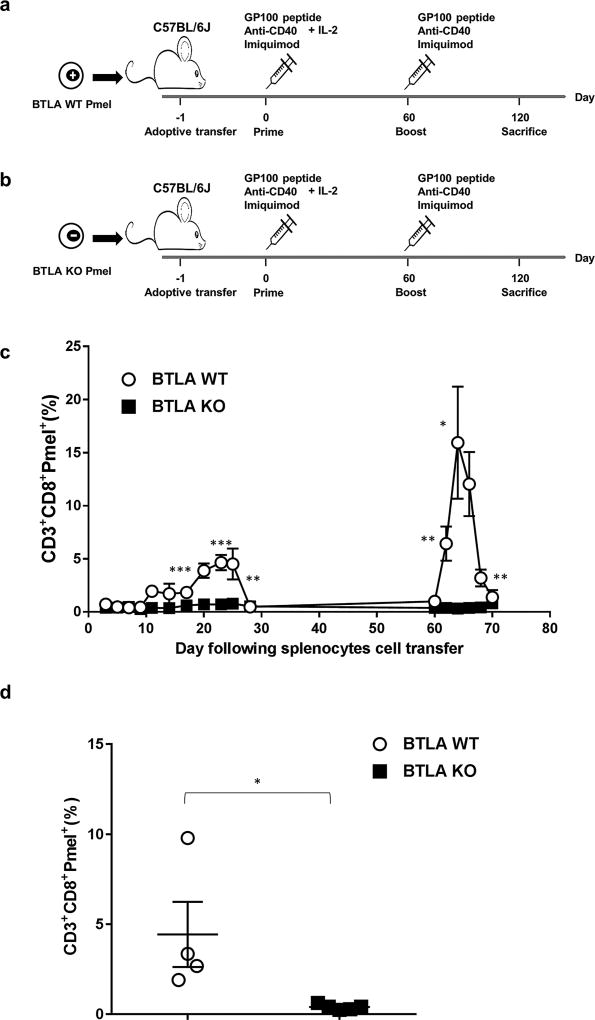
Figure 4. Defective memory recall response of BTLA deficient T cells.
Schematic diagram depicting experimental design of mouse model for vaccination. 0.5 × 106 (a) Pmel-1 Thy 1.1. wild type splenocytes or (b) Pmel-1 BTLA KO splenocytes were adoptively transferred into C57BL/6 mouse recipients (i.v.) (n=5 mice per group). On the following day, the recipients were vaccinated with gp100 peptide (100µg) together with anti-CD40 (50 µg) and imiquimod (50 mg). Recombinant human IL-2 at 1.2 ×106 IU was administered once, and 6 × 105 IU twice daily for the next 2 days (i.p.). Peripheral blood was collected every other day to determine the frequency of circulating Pmel-1 Thy 1.1 T cells. On day 60, the mic e were vaccinated with gp100 peptide, and peripheral blood was collected every other day until Pmel-1 T cells were no longer detected. On day 120, the mice were euthanized, and spleens were collected to determine the presence of Pmel-1 Thy 1.1 T cells. (c) Plot graph depicts the percentage of CD3+CD8+Pmel+ T cells in the peripheral blood following the priming (first peak from day 15 to day 30) and boosting (second peak from day 60 to 70). The frequency of CD3+CD8+Pmel+T cells was significantly higher in C57BL/6 mouse recipients receiving Pmel-1 Thy 1.1 wild type splenocytes as compared to those receiving Pmel-1 Thy 1.1 BTLA KO splenocytes. (d) Comparison of the percentage of CD3+CD8+Pmel+ T cell in spleen on day 120 of mice receiving either Pmel-1 Thy 1.1 wild type splenocytes or Pmel-1 Thy 1.1 BTLA KO splenocytes. Pmel-1 Thy 1.1 BTLA KO splenocytes failed to develop memory recall response following vaccination and boosting.