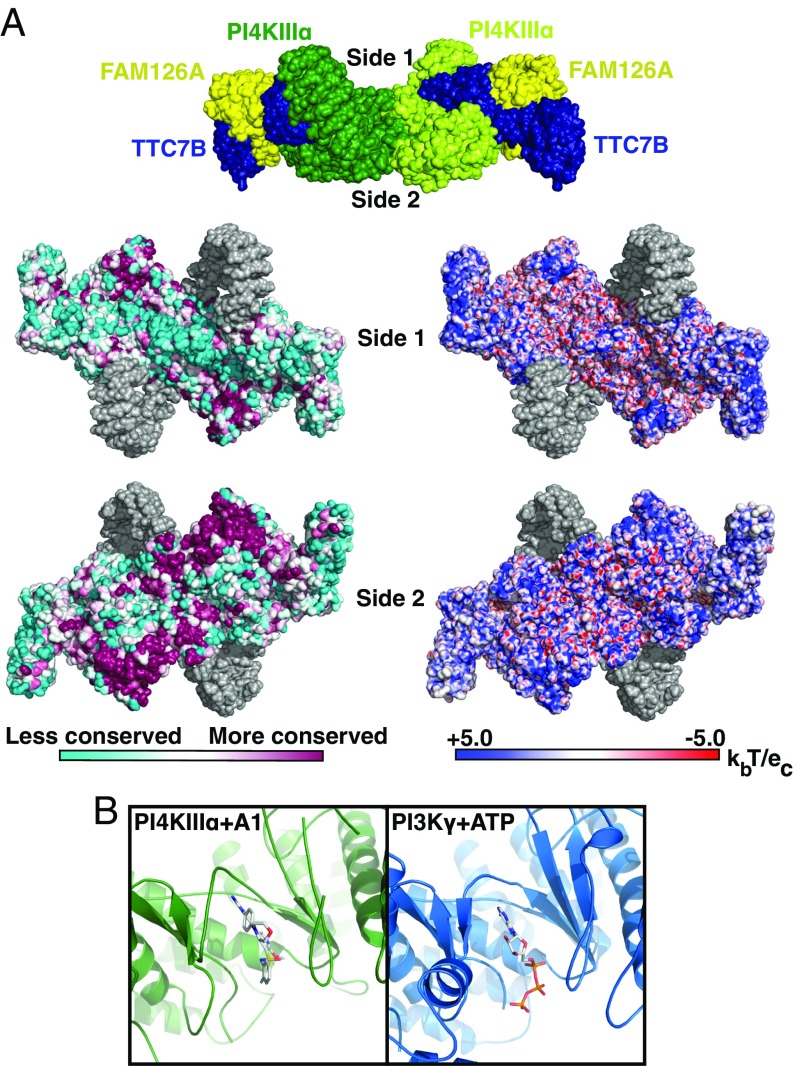
Fig. 4.
PI4KIIIα complex interacts with the plasma membrane via a large conserved surface that orients the catalytic domains toward substrate. (A, Top) Surface representation of PI4KIIIα complex, colored by subunit, as in Fig. 1A. Surfaces designated side 1 and side 2 are indicated by labels. (A, Lower Left) Side 1 and side 2 surfaces of PI4KIIIα complex, colored by conservation. Regions built as polyalanine helices are indicated in gray. (A, Lower Right) Side 1 and side 2 surfaces of PI4KIIIα complex, colored by surface charge. Regions built as polyalanine helices are indicated in gray. (B) Active site structures of PI4KIIIα + A1 inhibitor and PI3Kγ + ATP (PDB ID code 1E8X) are shown after structural alignment for comparison and oriented such that the putative membrane-binding surface of PI4KIIIα lies directly below the indicated view. In this orientation, the PI-binding site of each molecule lies directly below the bound ligand in each molecule.