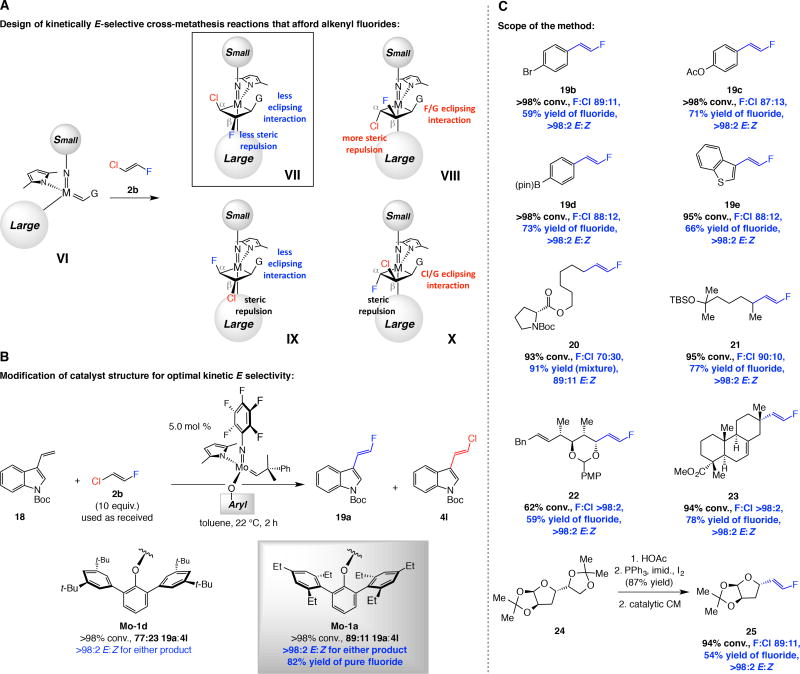
Fig. 4. Preparation of E-alkenyl fluorides by catalytic cross-metathesis.
(A) Mechanistic analysis that serves as the basis for the development of the catalytic processes. (B) Examination of a model process and identification of an effective complex. (C) With Mo-1a and commercially available E-1-chloro-2-fluoroethene 2b an assortment of E-1,2-alkenyl fluorides can be synthesized efficiently and with E:Z ratios that are typically >98:2. Yields are for purified products. Ac = acetyl. pin = pinacolato. Bn = benzyl. PMP = p-methoxyphenyl. Boc = tert-butoxycarbonyl. See the Supplementary Materials for details.