Abstract
The modulation of macrophage phenotype from pro-inflammatory (M1) to tissue healing (M2) via exogenous addition of interleukin-4 (IL-4) facilitates osteogenesis; however, the molecular mediators underlying this phenomenon remain unknown. This study characterizes the IL-4-dependent paracrine crosstalk between macrophages and osteoprogenitors and its effect on osteogenesis in vitro. Primary murine M1 were co-cultured with MC3T3 cells (M1-MC3T3) in both transwell plates and direct co-cultures. To modulate M1 to M2, M1-MC3T3 were treated with IL-4 (20ng/mL) at day 3 after seeding (M1+IL-4-MC3T3). Selected molecular targets were assessed at days 3 and 6 after seeding at protein and mRNA levels. Mineralization was assessed at day 21. Transwell M1+IL-4-MC3T3 significantly enhanced the secretion of CCL2/MCP-1, IGF-1 and to a lesser degree, CCL5/RANTES at day 6. At day 3, alkaline phosphatase (Alpl) was up-regulated in direct M1-MC3T3. At day 6, Smurf2 and Insulin growth factor-1 (Igf-1) were down-regulated and up-regulated respectively in direct M1+IL-4-MC3T3. Finally, M1+IL-4-MC3T3 increased bone matrix mineralization compared with MC3T3 cells in transwell, but this was significantly less than M1-MC3T3. Taken together, macrophage subtypes enhanced the osteogenesis in transwell setting and the transition from M1 to M2 was associated with an increase in bone anabolic factors CCL2/MCP-1, CCL5/RANTES and IGF-1 in vitro.
Keywords: CCL2/MCP-1, CCL5/RANTES, macrophages, insulin growth factor-1, osteogenesis
INTRODUCTION
Tissue injury activates the characteristic host response featured by inflammation followed by tissue-specific repair(1)–(4). Macrophages are multifunctional myelomonocytic derived-cells, that orchestrate inflammation and participate in tissue repair, leading either to full restitution of a morphological and functional tissue (regeneration)(2),(4) or to incomplete repair via a dysmorphic and non-functional scar (fibrosis)(2)–(4). Thus, macrophages have been proposed as a “master switch” between inflammation, tissue regeneration and scar formation(2). In this line, modulation of the macrophage-driven inflammatory step after injury is a potential therapeutic approach for regenerative medicine.
Immediately after bone injury, naive macrophages (M0) are recruited into the injured site and subsequently activated by local environmental cues. At the injury site, the release of danger signal molecules induces classical activation of macrophages. This involves phenotypic and functional changes acquiring the pro-inflammatory phenotype (M1) by activating Nuclear Factor-kappa B (NF-κB), the master pro-inflammatory transcription factor. Activation of NF-kB leads to production of chemokines and cytokines, including Macrophage-Chemoattractant Protein (MCP-1 also known as CCL2), Regulated on Activation Normal T Cell Expressed and Secreted (RANTES, CCL5), Tumor Necrosis Factor alpha (TNF-α), Interleukin (IL)-1β, IL-6 and IL-8 among others. These factors mediate the acute inflammatory response(5). With the disruption of local vasculature, bone injuries also represent an acute ischemic event for the tissue(6). Hypoxia induces the homing of hematopoietic-, mesenchymal-, and endothelial stem cells and progenitors from local and systemic reservoirs to the injured site(6). Indeed, injured tissues activate the Hypoxia Inducible Factor (HIF)-1/Stromal Cell-Derived Factor (SDF)-1 axis, which also activates NF-κB, thus synergizing to the release of pro-inflammatory mediators(6).
Once pro-inflammatory signals are cleared out and the recruitment of inflammatory precursors subsequently aborted (after approximately 72 hours), macrophages are reprogrammed to an anabolic phenotype, initiating the resolution phase of inflammation, a central step in re-establishing tissue homeostasis(7). Accordingly, macrophages are alternatively activated to the anabolic macrophage phenotype (M2), characterized by the secretion of the anti-inflammatory cytokines IL-4, IL-10, IL-1ra and growth factors such as vascular endothelial growth factor or VEGF, platelet-derived growth factor-BB or PDGF-BB and insulin growth factor-1 or IGF-1(8)–(10). M2 and recruited stromal stem cells contribute to the formation of a fibro-vascular granulation tissue ultimately resulting in local bone formation(4).
The advantages of sequential transition from M1 to M2 to tissue regeneration, rather than complete abolition of the M1-driven inflammation were recently investigated(8),(11),(12). We reported the improvement of osteogenesis by modeling the physiological transition from acute inflammation to tissue regeneration in vitro by modulating macrophage phenotype from M1 to M2-like cells via administration of M2 polarizing IL-4 at day 3 of direct co-culture(12). However, the necessity of cell-to-cell contact and details of the paracrine crosstalk amongst cells of the macrophage and osteoprogenitor lineages underlying this transition remain poorly understood. In this line, we hypothesize that the exogenous addition of IL-4 to M1-preosteoblast co-cultures will promote the secretion of both inflammatory chemokines and cytokines (CCL2/MCP-1, CCL5/RANTES, IL-1β and IL-10) and tissue anabolic growth factors (VEGF, PDGF-BB and IGF-1). The aim of this study was to characterize the paracrine crosstalk established between these cell lineages during the resolution phase of inflammation in vitro. Also, this study aimed to correlate the secretion of these factors with bone matrix mineralization, the last event in osteogenesis. We demonstrate that M1-, M1+IL-4- and M2- macrophages promote MC3T3-osteogenesis also in a transwell co-cultures by the paracrine crosstalk that involves at least CCL2/MCP-1, CCL5/RANTES and IGF-1 in vitro.
MATERIALS AND METHODS
Experimental design
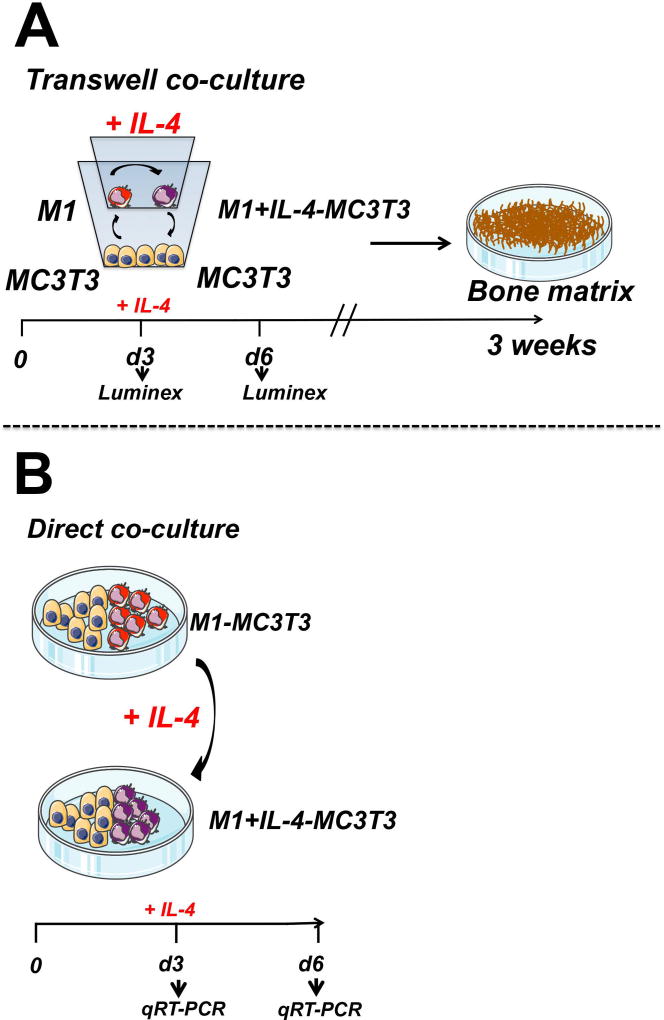
MC3T3 cells were co-cultured with M1 macrophages in both transwell (Figure 1A) and standard culture plate systems (Figure 1B). Seventy-two hours after seeding, IL-4 was added to the co-cultures to polarize the M1 to the M2 phenotype according to an established protocol(12) (Figure 1A and 1B).
Figure 1. Approaches to assess the effect of induced transition from M1 to M2 phenotype via added IL-4 on osteogenesis by MC3T3 cells.
a) Transwell co-culture of M1 and MC3T3. IL-4 was administered at day 3 after seeding to modulate M1 into M1+IL-4 macrophages. Then, mineralization of the bone matrix was evaluated at week 3 (endpoint). b) Relative expression of osteogenic-, inflammatory- and growth factors-related genes was assessed at days 3 and 6 after seeding from direct co-culture of M1-MC3T3 with or without IL-4 by qRT-PCR
Macrophage isolation and polarization
Institutional guidelines for the care and use of laboratory animals were carefully followed in all aspects of this study. Murine bone marrow macrophages were isolated as described by Pajarinen(13). Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) approved this isolation protocol. Frozen macrophages were thawed in T-175 culture flasks (8 × 106 cells/flask). Confluent macrophages were polarized to M1 or M2 phenotype by 24 hours exposure to basal medium (RPMI 1640, 10% heat inactivated fetal bovine serum (FBS), 1% antibiotic/antimycotic (all from Life Technologies, Carlsbad, CA), and 30% L929 conditioned medium (LCM)), with 100 ng/mL lipopolysaccharide (LPS) (Sigma Aldrich, St. Louis, MO), or with 20 ng/mL IL-4 (R&D Systems, Minneapolis, MN), respectively. Macrophage phenotype (TNFα for M1; CD206 for M2) was confirmed as established in our in our previous study(12)
Co-cultures
Transwell co-culture
The optimal ratio 1:1 of M1 or M2 together with MC3T3 cells was defined in a pilot experiment (data not shown) and by our previous study(12). Thus, 104 polarized M1 or M2 macrophages were seeded in the insert component of 24-well transwell co-culture system (0.4 µm) (Corning Inc., Lowell, MA, US) in mixed medium (1:1 ratio mix of minimum essential medium [MEMα; Life Technologies] and RPMI 1640 with 10 % FBS, 5 % LCM, 1 % antibiotic/antimycotic, 1 % GlutaMAX, 50 µg/ml l-ascorbic acid [Sigma-Aldrich], 10 mM β-glycerophosphate [Sigma-Aldrich], and 10 nM dexamethasone [Sigma-Aldrich]). (Figure 1A). Then, 104 MC3T3-E1 subclone 4 cells (104/well; ATCC, Manassas, VA) were seeded in the bottom plate of 24-well transwell plate in mixed medium (Figure 1A). Seventy-two hours after seeding, IL-4 was added to the M1-MC3T3 transwell co-cultures to reach a final IL-4 concentration of 20 ng/mL (Figure 1A). For controls, single M1, M2 and MC3T3 cells (104/well) were plated in the transwell system in MC3T3 growth medium (MEMα, 10% FBS, and 1% antibiotic/antimycotic) or mixed medium, with or without IL-4 administration at 72 hours. Media was changed twice a week for 3 weeks.
Direct co-culture
Macrophages were polarized prior to plating as described above. For untreated M1-MC3T3 co-cultures, M1 macrophages (104/well) were seeded simultaneously with MC3T3 cells in 24-well plates in mixed medium (Figure 1B). For IL-4 treated M1-MC3T3 co-culture (M1+IL-4-MC3T3), M1 and MC3T3 co-cultures were set up as described above. Then, IL-4 was administered once at day 3 after seeding to reach the final concentration of 20 ng/mL (Figure 1B). For controls, MC3T3 cells (104/well) were plated in 24-well plates in MC3T3 growth medium or mixed medium, with or without IL-4 administration at 72 hours. Media was changed twice a week for 3 weeks.
RNA isolation and qRT-PCR
After 3 and 6 days of cocultures, RNA was extracted using Qiagen’s RNeasy Mini Kit (Valencia, CA) and reverse transcribed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). qRT-PCR was performed using TaqMan Gene Expression Master Mix and 18s, Tnf-α, Cd206, runt related transcription factor 2 (Runx2), alkaline phosphatase, liver/bone/kidney (Alpl), SMAD specific E3 ubiquitin protein ligase 2 (Smurf2), Transcriptional Co-Activator With PDZ-Binding Motif (Taz), Insulin growth factor-1 (Igf-1) and Vascular endothelial growth factor (Vegfa) probes on an ABI 7900HT Sequencing Detection System (all from Applied Biosystems). The GenBank accession numbers of the probe sequences are 18s: NR_003278, Tnf-α: NM_013693 and NM_001278601, Cd206: NM_008625 XM_001003164 XM_001003168, Runx2: NM_001146038, Alpl: NM_007431, Smurf2: NM_025481 XM_126673, Taz (Wwtr1): NM_001168281, Igf-1: NM_001111274, Vegfa: NM_001025250. 18s rRNA was the internal control. Relative gene expression was quantified with the comparative Ct method(14).
Luminex
Secreted levels of inflammatory chemo and cytokines (CCL2/MCP-1, CCL5/RANTES, IL-1β and IL-10) and growth factors (VEGF, PDGF-BB and IGF-1) were assessed from day 3 and day 6 co-culture supernatants by Magnetic Luminex screening Assay (R&D Systems Inc.; Minneapolis, MN) following manufacturer’s instructions.
Alizarin Red staining
Three weeks after seeding, co-cultures were stained with Alizarin Red (pH 4.1, Sigma-Aldrich) for analysis of bone matrix formation. Plates were scanned with Perfection 1640SU (Epson, Long Beach, CA). Staining was eluted with 10% cetylpyridinium chloride (Sigma-Aldrich) and absorbance was measured at 562 nm.
Statistical analysis
Analyses were performed with GraphPad Prism version 6.04 (San Diego, CA). Unpaired t-test was used to analyze the difference between groups of two. Analyses for more than two groups were conducted with a one-way ANOVA followed by Tukey’s multiple comparisons test to compare each group (untreated co-culture experiment) or Dunnett’s multiple comparisons test to compare to the control group (IL-4 treated co-culture experiment). P<0.05 was considered statistically significant. Data are the results of at least three independent experiments and are expressed as mean ± standard deviation of the mean (SD), where *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
RESULTS
Added IL-4 modulates M1 into M2-like phenotype in direct co-cultures with MC3T3
To confirm the successful modulation of macrophage phenotype by added IL-4(12), the expression of Tnf-α and Cd206 was assessed by qRT-PCR 3 days after the addition of IL-4 to the direct co-cultures at day 6. As expected, the addition of IL-4 to the cultures resulted to downregulation of M1 related Tnf-α (p<0.05) and upregulation of M2 related Cd206 (p<0.01) (Figure 1 in Supplementary data).
M0, M1 and M2 macrophages promote osteogenesis in transwell co-cultures
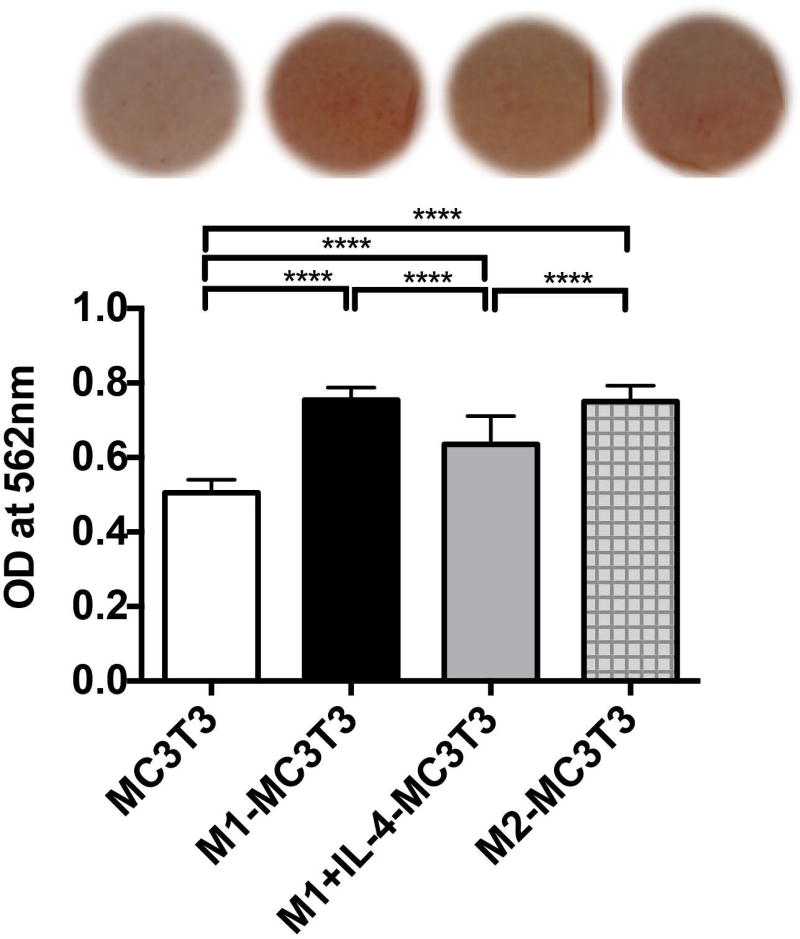
We previously determined that the M0, M1 and M2 macrophages at 1:1 ratio to MC3T3 significantly increased osteogenesis by MC3T3 in transwells (p<0.0001) in a pilot experiment (Data not shown). Subsequently, to determine how the transition from M1 to M2 via administration of exogenous IL-4 influenced osteogenesis by MC3T3 in transwell system, we evaluated the mineralization of bone matrix, using the same methods. While either M1, M1+IL-4 and M2 enhanced osteogenesis by MC3T3 compared with single cultured MC3T3 (p<0.00001), M1+IL-4 cells exhibited a significantly smaller increase in matrix mineralization than those exerted by M1 (p<0.00001) and M2 (p<0.00001) on MC3T3 cells (Figure 2).
Figure 2. Mineralization of bone matrix by single MC3T3, M1-MC3T3 with or without IL-4 and M2-MC3T3 co-cultures was assessed in transwell by Alizarin Red assay at week 3.
(OD) Optical density, ****p<0.0001
Macrophages and addition of IL-4 impacted the levels of CCL2, CCL4, IFG-1, and VEGF in transwell co-cultures
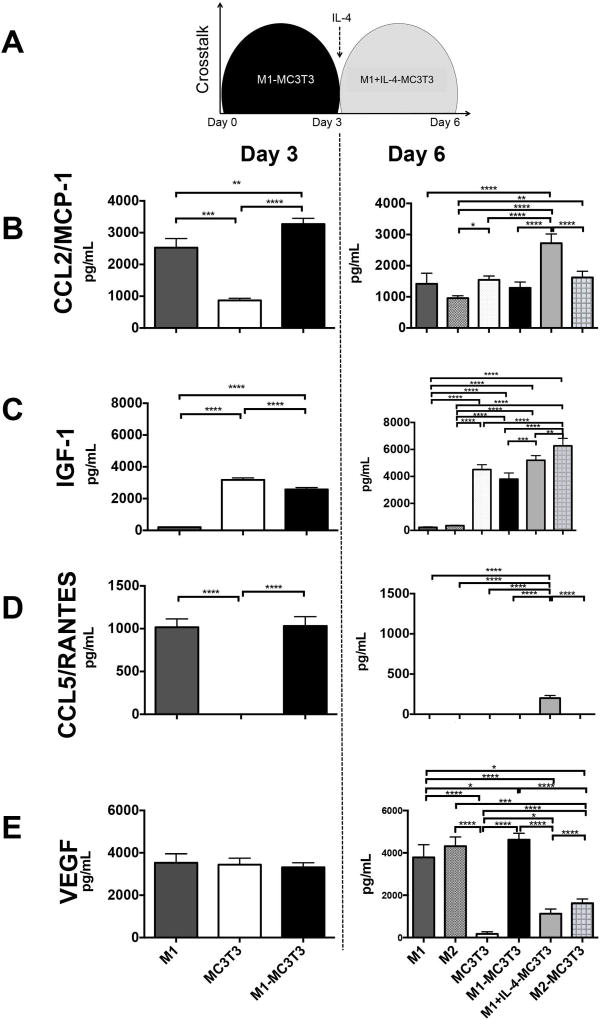
To characterize the crosstalk between M1 and MC3T3 and the effect of IL-4 on the co-cultures during the transition to M2-like (M1+IL-4) phenotype, we assayed the secreted-levels of selected chemokines, cytokines and growth factors from the transwell co-culture supernatant at day 3 (for M1-MC3T3) and day 6 (for M1+IL-4-MC3T3) (Figure 3). Although we assayed a total of 7 analytes, only 4 of them (CCL2/MCP-1, IGF-1, CCL5/RANTES and VEGF) were elevated in the culture supernatants.
Figure 3. Added IL-4 promotes the secretion of CCL2/MCP-1, IGF-1 and CCL5/RANTES.
a) Protein levels from M1-MC3T3 and IL-4 treated M1-MC3T3 (M1+IL-4-MC3T3) transwell co-cultures were assayed from transwell supernatants at days 3 and 6 after seeding respectively by Luminex. b–e) Day 3 (left panels) and day 6 (right panels) CCL2/MCP-1, IGF-1, CCL5/RANTES and VEGF protein secretions were quantified by Luminex, respectively *p<0.05; **p<0.01; ***p<0.001 and ****p<0.0001
CCL2/MCP-1
At day 3 (Figure 3B, left panel) we observed that M1-MC3T3 co-cultures secreted a significantly high level of CCL2/MCP-1 compared with single M1 (p=0.0054) or MC3T3 cultures (p<0.00001). The main source of CCL2/MCP-1 was M1 compared with MC3T3 (p=0.0001). At day 6 (Figure 3B, right panel) the secreted levels of CCL2/MCP-1 were globally reduced compared with those observed at day 3. In fact, single M1 and MC3T3 cultures, and M1- and M2-MC3T3 co-cultures secreted similar levels of CCL2/MCP-1. However, in IL-4 treated M1-MC3T3 cells (M1+IL-4-MC3T3) increased amounts of CCL2/MCP1 were detected and the concentrations were close to the ones observed at day 3 (p<0.0001).
IGF-1
At day 3, (Figure 3C, left panel) the main source of IGF-1 was MC3T3 compared with low level secreted by M1 (p<0.00001). IGF-1 secreted by M1-MC3T3 co-culture was significantly reduced compared to MC3T3 alone (p<0.00001). At day 6, the main source of IGF-1 was MC3T3 compared with M1 or M2 (p<0.0001) (Figure 3C, right panel). The IL-4 treatment (M1+IL-4-MC3T3) significantly enhanced the secretion of IGF-1 compared with untreated M1-MC3T3 (p=0.0004). Finally, the highest levels of IGF-1 were secreted by M2-MC3T3 cells compared with MC3T3 (p<0001), M1-MC3T3 (p<0.0001) and M1+IL-4-MC3T3 (p<0.005).
CCL5/RANTES
At day 3, both M1 and M1-MC3T3 groups enhanced the secretion of CCL5/RANTES compared with single MC3T3 cultures (p<0.0001) (Figure 3D, left panel). However, day 6 CCL5/RANTES levels were globally undetectable. Only the IL-4 treated M1-MC3T3 group (M1+IL-4-MC3T3) enhanced the secretion of CCL5/RANTES (p<0.0001) (Figure 3D, right panel).
VEGF
At day 3, M1, MC3T3 and M1-MC3T3 groups secreted similar high levels of VEGF (Figure 3E, left panel). At day 6, MC3T3 significantly decreased (p<0.0001) the level of VEGF compared with M1, M2 and M1-MC3T3 (Figure 3E, right panel). Moreover, the IL-4 treated M1-MC3T3 group (M1+IL-4-MC3T3) significantly reduced the secretion of VEGF compared with M1-MC3T3 (p<0.0001) and M2-MC3T3 (p<0.0001) (Figure 3E, right panel).
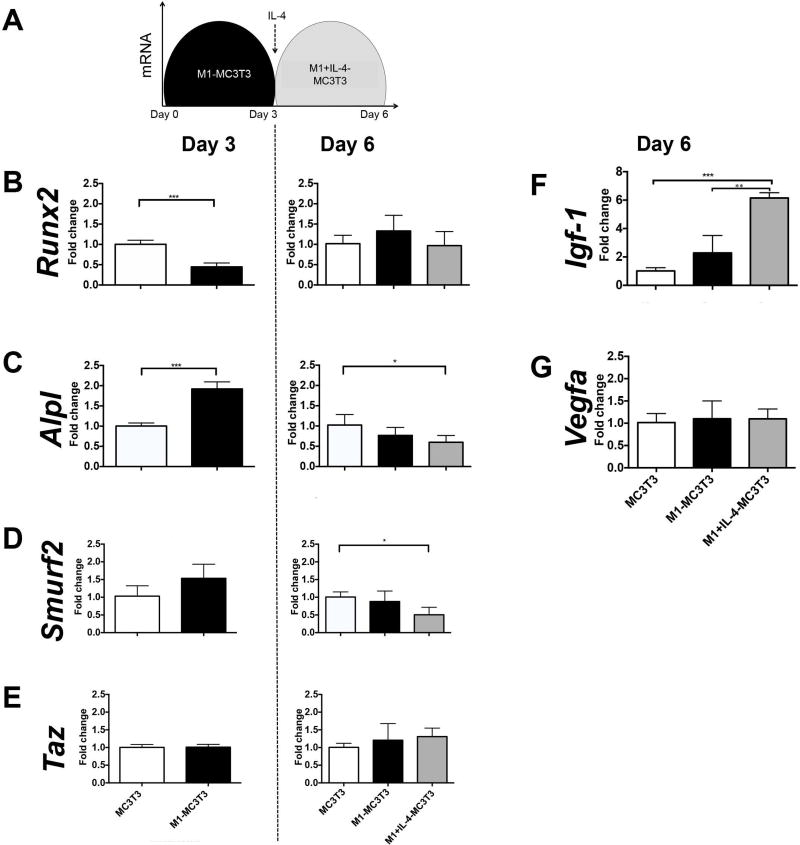
Addition of IL-4 triggers pro-osteogenic signals by down-regulating Smurf2 and up-regulating Igf-1 in direct co-cultures
To further characterize the mechanisms of M1 macrophage and IL-4 induced increase in osteogenesis in direct co-cultures, we assessed the relative expression of both the classic osteogenic-related (Runx2 and Alpl) and the NF-kB-related (Smurf2 and Taz) genes from IL-4 untreated and treated (M1+IL-4-MC3T3) M1-MC3T3 direct co-cultures at days 3 and 6 by qRT-PCR. Using the same methods, we characterized the mRNA relative expression of growth factors Igf-1 and Vegfa at day 6 (Figure 4). While Runx2 was down regulated by M1-MC3T3 at day 3 compared with single MC3T3 culture (p<0.001), no difference between M1-MC3T3 and M1+IL-4-MC3T3 groups was detected at day 6 (Figure 4B). Alpl was up regulated at day 3 by M1-MC3T3 (p<0.001), and down regulated by M1+IL-4-MC3T3 at day 6 (p<0.05) compared with MC3T3 (Figure 4C). Smurf2 was up regulated by M1-MC3T3 at day 3 (p=0.09) and significantly down regulated by M1+IL-4-MC3T3 at day 6 (p<0.05) (Figure 4D). No differences were registered in Taz expression at days 3 and 6 (Figure 4E). Interestingly, M1+IL-4-MC3T3 up regulated Igf-1 (p<0.001) at day 6 compared with both single MC3T3 and M1-MC3T3 (Figure 4F). Finally, no difference was observed in Vegfa expression at day 6 (Figure 4G).
Figure 4. IL-4 triggers pro-osteogenic signals by down-regulating Smurf2 and up-regulating Igf-1.
a) mRNA relative expression were determined from cell lysate by qRT-PCR at day 3 and 6 of direct co-culture; b–c) Day 3 (left panels) and day 6 (right panels) osteogenic related genes (Runx2 and Alpl); d-e) Day 3 (left panels) and day 6 (right panels) NF-kB related genes (Smurf2 and Taz); f) Day 6 Igf-1 and G) Day 6 Vegfa *p<0.05; **p<0.01 and ***p<0.001
DISCUSSION
Bone injuries represent tissue damaging and ischemic insults that trigger both inflammatory and hypoxic responses(15). Inflammatory and bone cells establish highly coordinated temporal- and spatially-dependent crosstalk in order to fully reconstitute the oxygen level, eliminate cell and tissue byproducts and facilitate bone repair leading to either bone regeneration or fibrosis(4). Recently, we established the importance of a transient M1-pro inflammatory period followed by IL-4 induced and M2-driven anti-inflammatory period to optimize osteogenesis by MC3T3 in direct co-cultures. Indeed, this study represents an in vitro model of the accelerated resolution phase of inflammation. As the mechanisms underlying this phenomenon remain unclear, we characterized the crosstalk between M1-, M1+IL-4- and M2-MC3T3 in an early stage of co-culture (days 3 and 6) using the transwell and direct cell-cell interaction culture systems. Moreover, we associated these findings with osteogenesis at week 3 in the same co-culture conditions. In this study, we first determined that 104 macrophage subsets seeded in the insert and 104 MC3T3 seeded in the bottom well at a 1:1 ratio were able to improve osteogenesis at week 3 in the transwell system. These results suggest that the impact of macrophages on MC3T3 osteogenic cell differentiation is mediated not only by direct cell contact as we demonstrated before, but also by paracrine factors secreted by macrophages. Then, as previously reported, we confirmed the polarizing effect of 20 ng/mL of IL-4, by observing down regulation of TNF-α and up-regulation of Cd206 by direct M1+IL-4-MC3T3 co-culture. We assessed the phenotype of co-cultured macrophages from cell lysates of direct macrophage-MC3T3 co-cultures as previously reported(12), due to the technical difficulties to detach them from the thin membrane of the transwell insert using chemical and physical methods. Also, our group has utilized the methods to polarize naive macrophages into M1 and M2 phenotypes(12),(13).
MC3T3 is a clonal non-transformed cell line established from newborn mouse calvaria, which is a representative model of the pre-osteoblastic stage of differentiation(16). MC3T3 cells exhibit alkaline phosphatase activity, synthesize collagen and mineralize in vitro. However, the generation of sub-clones after extended passages (>P30) make them lose their ability to mineralize(16)–(18). To prevent this reported issue, we considered MC3T3-E1 subclone four until passage 4 in our co-culture experiments(17),(18). Thus, the mineralization of the MC3T3-synthesized bone matrix was the most important endpoint outcome of this study. Here, we demonstrated that macrophage subsets regulate osteogenesis by MC3T3 cells by paracrine signals. In our previous study, we demonstrated that macrophage subsets regulate osteogenesis by juxtacrine signaling(12). Somewhat unexpectedly, transwell co-cultured M1+IL-4 macrophages were unable to optimize osteogenesis by MC3T3 cells at the same level of directly co-cultured M1+IL-4-MC3T3. This controversial result suggests the potential role of adhesion molecules (e.g.: E-selectin, VCAM-1) on osteogenesis(19),(20). While the control of early osteogenic programs during the transition from M1 to M2 involves both cell-cell contact and environmental cues, we propose that the tridimensional contact between macrophage subsets and MC3T3 cells is a crucial aspect.
To better understand the cross-talk between MC3T3 cells, macrophage subtypes and added IL-4 both in transwell and direct co-cultures, we determined the levels of selected cytokines with multiplex assay, as well as the expression of selected osteogenic factors with qRT PCR. Intriguingly, the pro-inflammatory environment (M1-MC3T3) activated both the anti-osteogenic (down-regulation of Runx2 and up-regulation of Smurf2) and, the pro-osteogenic pathways at day 3 (up-regulation of alkaline phosphatase). In this line, we previously reported that M1-MC3T3 secrets high levels of TNF-α as well as IL-1ra, the antagonist of IL-1β, at the same time point(12). Taken together, these findings are consistent with the anti-osteogenic effect of a pro-inflammatory environment created by M1 (TNF-α and IL-1β), by degrading Runx2 and up regulating both Smurf1 and Smurf2(21),(22). However, inflammation has also been reported as a promoter of osteogenesis. This paradoxical effect has been associated to the amount of TNF-α in the microenvironment(22),(23). Indeed, a low concentration of TNF-α promoted osteogenesis and mineralization and on the contrary, a high concentration decrease the same outcome(22)–(24). IL-4 added to the cultures at day 3 induced generally a pro-osteogenic effect by inducing bone anabolic signals (down-regulation of Smurf2 and increased secretion of CCL2/MCP-1, CCL5/RANTES and IGF-1).
As expected, M1-secreted CCL2/MCP-1 and CCL5/RANTES dominated the early inflammatory phase of co-culture (day 3) however, only CCL2/MCP-1 was significantly increased by M1+IL-4-MC3T3 co-culture (day 6). Otherwise, CCL5/RANTES peaked transiently at day 3, with globally low or undetectable levels at day 6. While CCL-2/MCP-1 and CCL5/RANTES are leukocyte chemoattractants mainly secreted by M1(5), CCL5/RANTES has been reported as responsible for the homing of endothelial stem cells during the initiation phase of angiogenesis in tissue engineered constructs(8),(25),(26). Specifically for bone tissue, an increased level of CCL2/MCP-1 transcript was strongly associated with the administration of the bone anabolic treatment (parathyroid hormone) in rats(27),(28). Also, increased expression of CCL2/MCP-1 was reported in periosteal cells at the early stage of fracture healing(29). On the other hand, the pro-osteogenic role of CCL5/RANTES was recently reported in human mesenchymal stem cells in vitro(26). Besides, CCL5/RANTES is a key regulator of bone remodeling in immune associated conditions such as rheumatoid arthritis or multiple myeloma(29). Taken together, these findings suggest that the first three days are a critical period to gather the stock of both inflammatory and vascular cell precursors for the following steps of bone repair.
The peak of CCL2/MCP-1 in M1+IL-4-MC3T3 cultures at day 6 can also be explained by a positive regulatory loop established between basal levels of CCL2/MCP-1 and the exogenous administration of IL-4. Gu et al (2000) and Li et al (2015) reported that CCL2/MCP1 promotes both the secretion of endogenous IL-4(30) in vivo and the M2 polarization of tumor-associated macrophages (TAM) in vivo(31). Alternatively, the low levels of CCL2/MCP-1 secreted by MC3T3 at day 3 and at day 6 could be explained by the presence of pro-inflammatory cytokines in culture mixed media, which contains LCM(32). Similarly, Bussard et al (2010), reported that the osteoblastic cell lines are important sources of inflammatory signals when they are in a tumor microenvironment(33).
IGF-1 is an anabolic growth factor that regulates skeletal modeling(34), bone remodeling(3),(34),(35) and fracture healing(36). IGF-1 is similarly expressed by both M1 and M2 macrophages with a transient up regulation during the resolution phase of inflammation(11). Mechanistically, IGF-1 acts on MC3T3 cells by increasing protein synthesis via the autocrine loop of AKT/m-TOR pathway(37). We observed that MC3T3 cells, the most important source of IGF-1, decrease IGF-1 levels when they were co-cultured with M1 and on the contrary, they raised IGF-1 levels when they were co-cultured with any IL-4 polarized M2 phenotype (M1+IL-4 and M2). These findings confirmed that M1 and M2 regulate negatively and positively respectively, the synthesis and secretion of IGF-1 by MC3T3 cells. We hypothesize that the exogenous administration of IL-4 to M1 and the endogenous secretion of IL-4 by M2(10) could be involved with this differential regulation of IGF-1 synthesis. In addition, Barret et al (2015) reported that autocrine signalization of IGF-1 contributes to the adoption of the full IL-4 dependent M2 phenotype(38).
VEGF secreted by both osteoblasts and macrophages is a known critical growth factor for fracture healing(4),(39). While VEGF has usually been associated to the tissue remodeling M2, recent studies showed that M1 is an important source of VEGF in vitro(10) and in tissue engineered constructs(8). Our findings confirmed that M1 with and without MC3T3 cells was the most stable source of VEGF at both time points and, that the role of MC3T3-secreted VEGF is only transient at day 3. High levels of M1-secreted VEGF were also described by Spiller et al (2014), using primary human blood-derived macrophage phenotypes seeded on a tissue-engineered scaffold(8). These findings could be associated with the important chemoattractant role exerted by VEGF on circulating monocytes(40), contributing to M1 functions. Further studies are needed to elucidate this relationship and the potential role of IL-4 on VEGF secretion.
In conclusion, we demonstrate that macrophage subtypes promote MC3T3 mediated bone formation also in a transwell setting. Adding IL-4 to the transwell co-cultures increased the secretion of bone anabolic factors including CCL2/MCP-1, CCL5/RANTES and IGF-1, but it was not effective in enhancing the overall bone formation in the absence of cell-to-cell contact. While this study suggests that CCL2/MCP-1, CCL5/RANTES, and IGF-1 participate in the crosstalk between macrophages and MC3T3 cells during the resolution phase of inflammation in vitro, further functional studies, e.g. with blocking antibodies are needed to elucidate the role of each of the above pathways on osteogenesis during the resolution of inflammation. The possible modulation of these detected molecules - or their receptors - may lead to developing a targeted therapy to optimize the bone healing in clinical settings.
Supplementary Material
Figure 1 in Supplementary data. Modulation from M1- to M1+IL-4 phenotype in IL-4 treated and untreated M1-MC3T3 co-cultures administrated at day 3. Phenotypes were assessed by mRNA expression of Tnf-α for M1 phenotype and Cd206 for M2 phenotype (*) p<0.05 and (**) p<0.01
Acknowledgments
We thank K.P. Jensen for technical assistance with the Luminex assay. This work was supported by NIH grants 2R01AR055650 and 1R01AR063717 and Stanford University’s Ellenburg Chair in Surgery to SBG, and University of Chile-Conicyt Becas Chile Award to LC (CONICYT PAI/INDUSTRIA 79090016).
References
- 1.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–62. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–69. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 4.Loi F, Córdova LA, Pajarinen J, Lin T-H, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86:119–30. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, Goodman SB. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 7.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–74. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton DW, McManus LM, Gelfond JA, Shireman PK. Temporal phenotypic features distinguish polarized macrophages in vitro. Autoimmunity. 2015;48:161–76. doi: 10.3109/08916934.2015.1027816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol Ther J Am Soc Gene Ther. 2015;23:1189–200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loi F, Córdova LA, Zhang R, Pajarinen J, Lin T-H, Goodman SB, Yao Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7:15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajarinen J, Tamaki Y, Antonios JK, Lin T-H, Sato T, Yao Z, Takagi M, Konttinen YT, Goodman SB. Modulation of mouse macrophage polarization in vitro using IL-4 delivery by osmotic pumps. J Biomed Mater Res A. 2015;103:1339–45. doi: 10.1002/jbm.a.35278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kartsogiannis V, Ng KW. Cell lines and primary cell cultures in the study of bone cell biology. Mol Cell Endocrinol. 2004;228:79–102. doi: 10.1016/j.mce.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and Characterization of MC3T3-E1 Preosteoblast Subclones with Distinct In Vitro and In Vivo Differentiation/Mineralization Potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 18.Yan X-Z, Yang W, Yang F, Kersten-Niessen M, Jansen JA, Both SK. Effects of continuous passaging on mineralization of MC3T3-E1 cells with improved osteogenic culture protocol. Tissue Eng Part C Methods. 2014;20:198–204. doi: 10.1089/ten.tec.2012.0412. [DOI] [PubMed] [Google Scholar]
- 19.Dohle E, Bischoff I, Böse T, Marsano A, Banfi A, Unger RE, Kirkpatrick CJ. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur Cell Mater. 2014;27:149–164. doi: 10.22203/ecm.v027a12. discussion 164–165. [DOI] [PubMed] [Google Scholar]
- 20.Ma B, Dohle E, Li M, Kirkpatrick CJ. TLR4 stimulation by LPS enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.2075. [DOI] [PubMed] [Google Scholar]
- 21.Kaneki H, Guo R, Chen D, Yao Z, Schwarz EM, Zhang YE, Boyce BF, Xing L. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281:4326–33. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Ghali O, Lencel P, Broux O, Chauveau C, Devedjian JC, Hardouin P, Magne D. TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009;84:499–504. doi: 10.1016/j.lfs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Lencel P, Delplace S, Pilet P, Leterme D, Miellot F, Sourice S, Caudrillier A, Hardouin P, Guicheux J, Magne D. Cell-specific effects of TNF-α and IL-1β on alkaline phosphatase: implication for syndesmophyte formation and vascular calcification. Lab Investig J Tech Methods Pathol. 2011;91:1434–42. doi: 10.1038/labinvest.2011.83. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Xu D, Liu Y, Zhang R, Lu L. The Effect of Tumor Necrosis Factor-α at Different Concentrations on Osteogenetic Differentiation of Bone Marrow Mesenchymal Stem Cells. J Craniofac Surg. 2015;26:2081–5. doi: 10.1097/SCS.0000000000001971. [DOI] [PubMed] [Google Scholar]
- 25.Suffee N, Hlawaty H, Meddahi-Pelle A, Maillard L, Louedec L, Haddad O, Martin L, Laguillier C, Richard B, Oudar O, Letourneur D, Charnaux N, Sutton A. RANTES/CCL5-induced pro-angiogenic effects depend on CCR1, CCR5 and glycosaminoglycans. Angiogenesis. 2012;15:727–44. doi: 10.1007/s10456-012-9285-x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y-C, Kao Y-T, Huang W-K, Lin K-Y, Wu S-C, Hsu S-C, Schuyler SC, Li L-Y, Leigh Lu F, Lu J. CCL5/RANTES is important for inducing osteogenesis of human mesenchymal stem cells and is regulated by dexamethasone. Biosci Trends. 2014;8:138–43. doi: 10.5582/bst.2014.01047. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JHM, Notterman DA, Partridge NC. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem. 2007;282:33086–97. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J Biol Chem. 2007;282:33098–106. doi: 10.1074/jbc.M611781200. [DOI] [PubMed] [Google Scholar]
- 29.Wu AC, Morrison NA, Kelly WL, Forwood MR. MCP-1 Expression Is Specifically Regulated During Activation of Skeletal Repair and Remodeling. Calcif Tissue Int. 2013;92:566–75. doi: 10.1007/s00223-013-9718-6. [DOI] [PubMed] [Google Scholar]
- 30.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 31.Li N, Qin J, Lan L, Zhang H, Liu F, Wu Z, Ni H, Wang Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol Ther. 2015;16:297–306. doi: 10.1080/15384047.2014.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege J-L, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bussard KM, Venzon DJ, Mastro AM. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J Cell Biochem. 2010;111:1138–48. doi: 10.1002/jcb.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng MHC, Lau KHW, Baylink DJ. Role of Osteocyte-derived Insulin-Like Growth Factor I in Developmental Growth, Modeling, Remodeling, and Regeneration of the Bone. J Bone Metab. 2014;21:41–54. doi: 10.11005/jbm.2014.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemmons DR. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest. 2004;113:25–7. doi: 10.1172/JCI200420660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidmaier G, Wildemann B, Gäbelein T, Heeger J, Kandziora F, Haas NP, Raschke M. Synergistic effect of IGF-I and TGF-beta1 on fracture healing in rats: single versus combined application of IGF-I and TGF-beta1. Acta Orthop Scand. 2003;74:604–10. doi: 10.1080/00016470310018036. [DOI] [PubMed] [Google Scholar]
- 37.Bakker AD, Gakes T, Hogervorst JMA, de Wit GMJ, Klein-Nulend J, Jaspers RT. Mechanical Stimulation and IGF-1 Enhance mRNA Translation Rate in Osteoblasts Via Activation of the AKT-mTOR Pathway. J Cell Physiol. 2016;231:1283–90. doi: 10.1002/jcp.25228. [DOI] [PubMed] [Google Scholar]
- 38.Barrett JP, Minogue AM, Falvey A, Lynch MA. Involvement of IGF-1 and Akt in M1/M2 activation state in bone marrow-derived macrophages. Exp Cell Res. 2015;335:258–68. doi: 10.1016/j.yexcr.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Saran U, Gemini Piperni S, Chatterjee S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109–17. doi: 10.1016/j.abb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 40.De Palma M. Partners in crime: VEGF and IL-4 conscript tumour-promoting macrophages. J Pathol. 2012;227:4–7. doi: 10.1002/path.4008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 in Supplementary data. Modulation from M1- to M1+IL-4 phenotype in IL-4 treated and untreated M1-MC3T3 co-cultures administrated at day 3. Phenotypes were assessed by mRNA expression of Tnf-α for M1 phenotype and Cd206 for M2 phenotype (*) p<0.05 and (**) p<0.01