Figure 6.
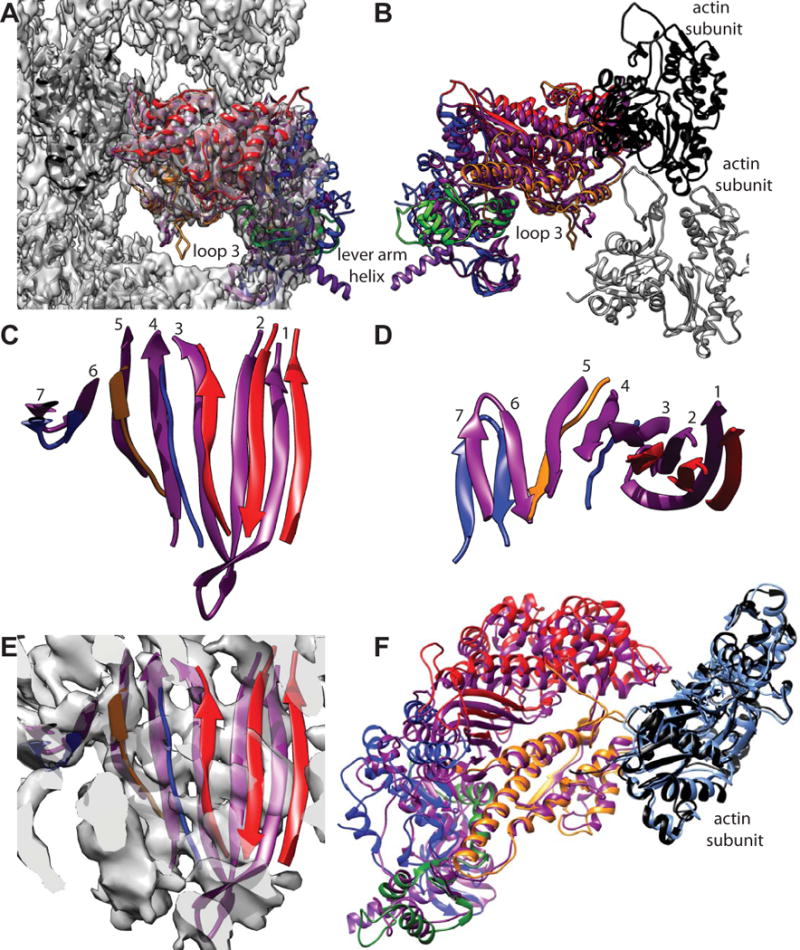
Comparison of acto-smMD with acto-skMD. (A) The two reconstructions shown with the acto-smMD reconstruction. The acto-skMD was aligned to the acto-smMD using the actin subunit coordinates from the acto-skMD atomic model (PDB 5H53), which is colored black, to drive the alignment. There is little difference between the actin subunit atomic models from the two reconstructions. The smMD atomic model is colored purple. The acto-skMD atomic model is colored according to the MD subdomains, which are N-terminal 25 kDa domain (blue), upper 50 kDa domain (red), lower 50 kDa domain (orange), converter domain (green) and the lever arm (magenta). Note that the smMD does not have the lever arm helix. (A) Both atomic models shown within the reconstruction envelope. Many features of the skMD atomic model fall outside of the density envelope of the smMD. The most obvious difference is the position of loop 3 (skeletal residues K567–F579), which falls clearly outside the reconstruction envelope. Loop 3 is part of the lower 50 kDa domain. (B) The atomic models of the skMD and the smMD shown with a pair of actin subunits, one black, the other gray. This view from the opposite direction from that of panel A. (C) Comparison of the transducer β-sheet with the smooth muscle structure shown in purple and the skeletal muscle sheet colored according to subdomain origin. Since the sheet itself is curved, the displacements for strands 1 and 2 are the most obvious. This view direction is from outside the MD looking in towards the actin-binding cleft. The relative displacement has the skeletal β-sheet to the side and on the outside of the smooth β-sheet (roughly looking from the top of panel F towards the bottom). (D) View looking down from the top of panel C. (E) Same view direction as panel C but with the reconstruction envelope showing. Note that the skMD β-sheet mostly falls outside of the corresponding density envelope. (F) View looking down the actin binding cleft showing the actin subunit atomic models from the two reconstructions as well as their MD atomic models. The actin atomic model from the acto-smMD reconstruction is shown in sky blue. Note that the helices of the lower 50 kDa domains overlap well, whereas features of the upper 50 kDa domains overlap poorly. The 25 kDa domains also overlap poorly.
