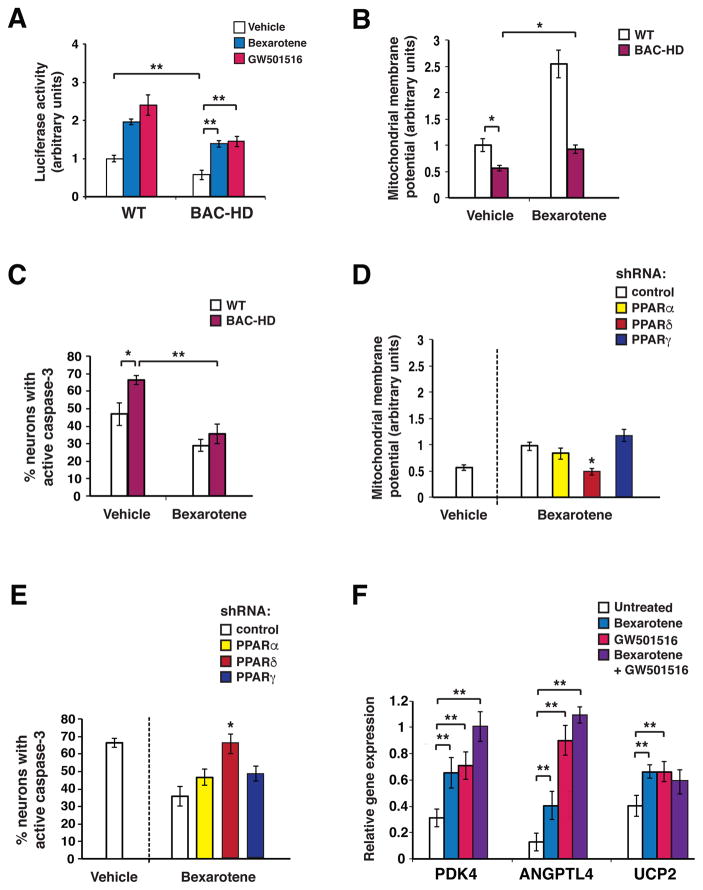
Figure 1. Bexarotene promotes PPARδ activation to ameliorate the neurotoxicity of mutant huntingtin.
(A) We measured 3X-PPRE luciferase reporter activity in primary cortical neurons from wild-type (WT) control mice or BAC-HD mice, co-transfected with Renilla luciferase vector, and treated with bexarotene (500 nM), GW501516 (100 nM), or vehicle. PPARδ activation in BAC-HD mouse neurons was repressed at baseline compared to WT mouse neurons. **P < 0.01, Student’s t-test. Bexarotene and GW501516 treatment promoted PPARδ activation in BAC-HD mouse neurons. **P <0.01, ANOVA with post-hoc Tukey test. n = 3 biological replicates; n = 3 technical replicates. Results were normalized to WT mouse neurons at baseline. (B) Mitochondrial membrane potential of primary cortical neurons from WT and BAC-HD mice, treated with vehicle or bexarotene (500 nM), was determined from the ratio of mitochondrial to cytosolic JC-1 fluorescence. *P < 0.05, Student’s t-test. n = 3 biological replicates; n = 3 technical replicates. Results were normalized to WT mouse neurons at baseline. Similar results were obtained using TMRM as the fluorescent probe (Fig. S1B). (C) We quantified active caspase-3 immunostaining of primary cortical neurons from WT and BAC-HD mice, treated with vehicle or bexarotene (500 nM) for 24 hours, and H2O2 (25 μM for 4 hours). *P < 0.05, **P < 0.01; Student’s t-test. n = 3 biological replicates; 30 to 50 cells were counted/experiment. (D) Mitochondrial membrane potential was measured in BAC-HD mouse primary cortical neurons, transfected with the indicated shRNA expression vector (control = scrambled shRNA), and treated with vehicle or bexarotene (500 nM). Mitochondrial membrane potential was determined from the ratio of mitochondrial to cytosolic JC-1 fluorescence. *P < 0.05; ANOVA with post-hoc Tukey test. n = 3 biological replicates; n = 3 technical replicates. Results were normalized to WT mouse neurons at baseline as in panel B. (E) We quantified active caspase-3 immunostaining of BAC-HD mouse primary cortical neurons, transfected with the indicated shRNA expression vectors, and treated with vehicle or bexarotene (500 nM) for 24 hours, and H2O2 (25 μM, for 4 hours). *P < 0.05; ANOVA with post-hoc Tukey test. n = 3 biological replicates; 30 to 50 cells were counted/experiment. (F) We performed RT-PCR analysis of RNA expression of the PPARδ target genes PDK4, Angptl4, and UCP2 in BAC-HD mouse primary cortical neurons, treated as indicated. **P < 0.01; ANOVA with post-hoc Tukey test. n = 6 independent experiments. Error bars = s.e.m.