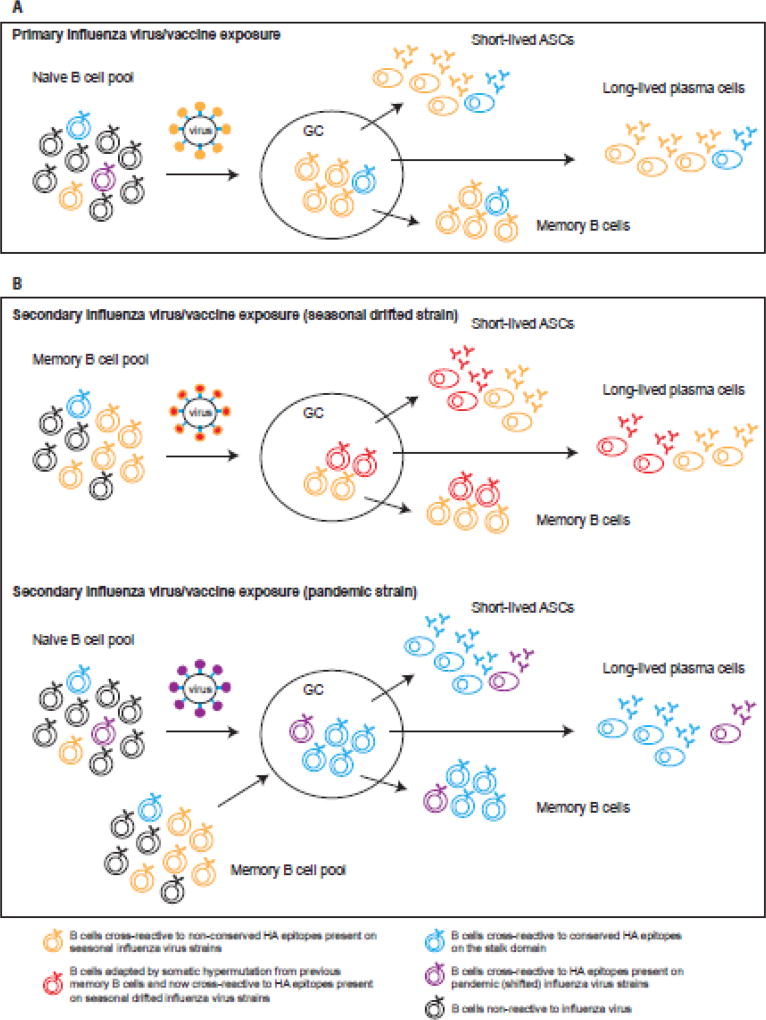
Figure 1. Antibody responses to influenza virus and antigenic seniority.
A. Primary exposure to an influenza virus (early in childhood) induces activation of influenza virus-reactive naive B cells. After antigen encounter and T cell help, naïve B cells enter a germinal center (GC) reaction where they class-switch and affinity mature by somatic hypermutation. The majority of the B cells will recognize epitopes on the immunodominant head domain of HA (in orange) but a few might recognize the more conserved stalk region (in blue). After 7–10 days, influenza-reactive short-lived antibody secreting cells (ASCs) or plasmablasts are present in circulation and secrete antibodies. The generation of influenzareactive memory B cells and long-lived plasma cells also occur from the GC and reflects the B cells that have been activated, with the majority of the antibody specificity being directed towards the HA head domain.
B. During secondary exposure to influenza virus/vaccine, memory B cells generated from the first exposure will be reactivated. However different outcomes will happen if the second exposure (infection or vaccination) is a seasonal influenza virus strain or a pandemic strain. In the case of a seasonal virus, antigenic drift happens from year to year resulting in small changes in the globular head. The pool of memory B cells generated from previous exposure will recognize these drifted epitopes on the head, cells will be reactivated and some of them will adapt in the GC by somatic hypermutation to increase binding to the drifted epitope (in red). Short-lived ASCs, long-lived plasma cells and memory B cells will be generated and the antibody specificity will be a mixture of the senior strain and the more junior strain epitopes (antigenic imprinting) but mainly targeting the head. Thus, previously encountered strains will be boosted by encounter with strains of the same subtype and over time, these responses accumulate. The less frequent memory B cells targeting conserved epitopes will not be boosted and will be diluted out. In the OAS model, the reduction in access to antigen because of pre-existing cross-reactive antibodies (epitope masking) is what would favor recall of memory over de novo activation of naïve B cells.
In the case of a pandemic influenza virus (shifted), the majority of the epitopes on the head domain are new (in purple) whereas conserved epitopes on the HA stalk remain the same. Cross-reactive memory B cells specific to the HA stalk (in blue) are being re-activated and now the product of the GC reaction is an antibody response targeting mainly the stalk domain of HA. Naïve responses to the novel epitopes on the head domain are also induced but most likely participate at a lower significance to the overall response.