FIG 12.
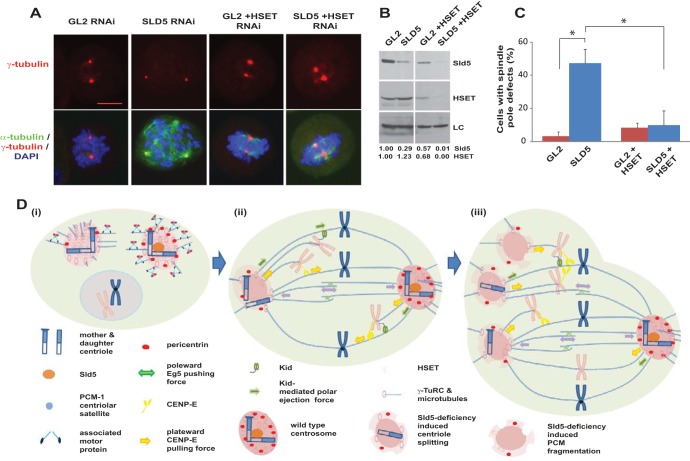
Inhibition of HSET prevents spindle pole fragmentation in Sld5-depleted cells. (A) HeLa cells transfected with control GL2, SLD5, or HSET siRNA, as indicated, with the combined concentration brought to 80 nM with GL2 siRNA. The cells were fixed and costained for α-tubulin (green) and γ-tubulin (red) and for DNA with DAPI (blue). Note that HSET depletion resulted in shortened or asymmetric spindle poles. Scale bar, 10 μm. (B) The transfected samples were immunoblotted with anti-Sld5 and anti-HSET antibodies to confirm RNAi depletion. LC, loading control showing equal protein loads in different lanes; the numbers indicate levels of Sld5 and HSET proteins in different samples relative to control GL2 siRNA-transfected cells. (C) Quantification of the spindle pole defects shown in panel A, represented as the means and SD of the results of three independent experiments, with more than 20 cells analyzed in each sample (*, P < 0.05). (D) Schematic representation of spindle pole fragmentation in the absence of Sld5. (i) Depletion of Sld5 leads to the dissipation of centriolar satellite protein PCM-1 in interphase cells, resulting in the loss of its known function of recruiting the centrosomal protein pericentrin. (ii and iii) During alignment of chromosomes in prometaphase, kinesin, CENP-E, and the chromokinesin Kid cause the unaligned chromosomes to migrate away from the spindle poles, exerting a significant pulling force on the centrosomes. While a wild-type centrosome (shown as the right pole) is able to resist this force, a Sld5-deficient centrosome (shown as the left pole) splits, forming monocentriolar or acentriolar spindle poles, as depicted in diagram iii. The minus-end-directed kinesin-14 motor protein, HSET, anchors the microtubules at the centrosomes.