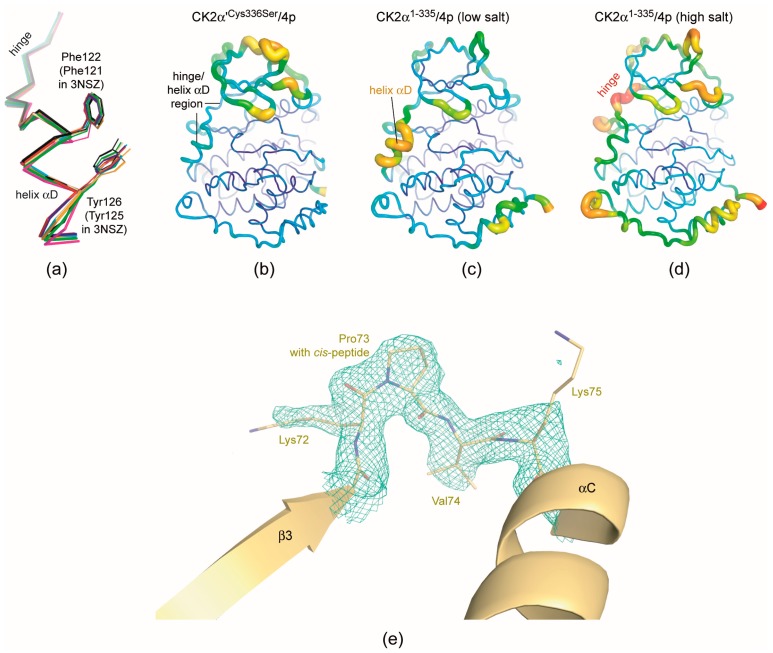
Figure 6.
Structural features of the CK2α′Cys336Ser/4p complex. (a) Overlay of the hinge/helix αD regions of the two CK2α′ protomers of this study (red and blue), of CK2α2−331 in 3NSZ [44] as a reference structure for the open hinge/helix αD conformation (black) and of all other CK2α′ chains available in the PDB (3OFM [31]: green; 3E3B [39]: magenta; 5M4U [38]: orange; 5M56-chain A: cyan; 5M56-chain B [38]: yellow); due to an insertion of one residue in the N-terminal segment of CK2α′ the numbering schemes of CK2α and CK2α′ are not identical: Phe121 and Tyr125 of CK2α are equivalent to Phe122 and Tyr126 of CK2α′. (b–d) Rainbow representation of the atomic B-factors in the CK2α′Cys336Ser/4p complex (b) and for comparison in the low-salt the CK2α1−335/4p complex (c) and in the low-salt the CK2α1−335/4p complex (d). Large B-factors (and thus high internal mobilities): red and thick; low B-factors (and thus low internal mobilities): blue and thin. (e) A cis-peptide bond between Lys72 and Pro73 in chain A of the CK2α′Cys336Ser/4p complex structure testified by the final electron density (cutoff level 1.0 σ).