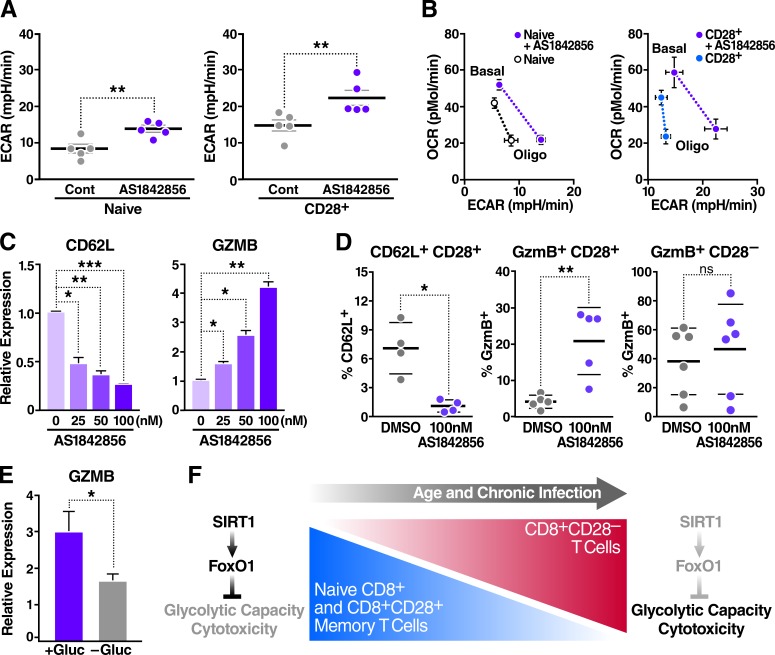
Figure 4.
FoxO1 inhibition reprograms CD8+ T cell metabolism and induces cytotoxicity. Metabolism of sorted human CD8+ T cell populations was assessed using an extracellular flux analyzer. (A) Glycolytic capacity of sorted human T cell populations treated with 100 nM AS1842856 for 48 h (n = 5, paired two-tailed Student’s t test). (B) Energy profile (OCR vs. ECAR) of T cell subsets treated as in A (shown is the mean of biological replicates, n = 5). (C) Gene expression was measured by qRT-PCR, normalized to RPL13A mRNA, and relative to untreated human CD8+ T cells with increasing doses of AS1842856 for 48 h (n = 3, paired two-tailed Student’s t test). (D) Surface marker analysis by flow cytometry after 72 h of incubation with 100 nM AS1842856 (n = 5, two-way ANOVA). (E) Gene expression after CD8+ T cell treatment with 100 nM AS1842856 ± glucose for 48 h, measured by qRT-PCR, and normalized to RPL13A mRNA and to untreated cells (n = 5, paired two-tailed Student’s t test). Data are mean ± SEM of individual donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001; mpH/min, milli-pH units per minute. (F) Model: a dynamic SIRT1–FoxO1 axis regulates metabolism and cytotoxicity in CD8+ T cells. This axis is lost in CD8+CD28– T cells, resulting in increased T cell glycolytic capacity and cytotoxicity.