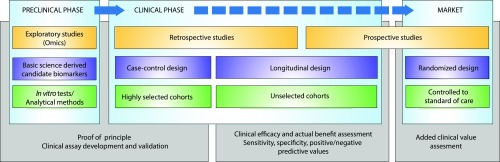
Figure 5.
Ideal pipeline for developing clinically usable and useful biomarkers in renal transplantation. The clinical assessment of medical devices is on the basis of completely different methods from those applied to medicinal products; consequently, new tools might be marketed after a very limited validation process, which may alter the capacity of the medical device to be reimbursed by National Health Insurance, with reimbursement being guided on the basis of assessments of the actual benefit and the added clinical value. Therefore, a strong multistep validation is required to avoid concerns that new diagnostic tests may increase health care expenditures and skepticism about their benefits in terms of improving clinical management.