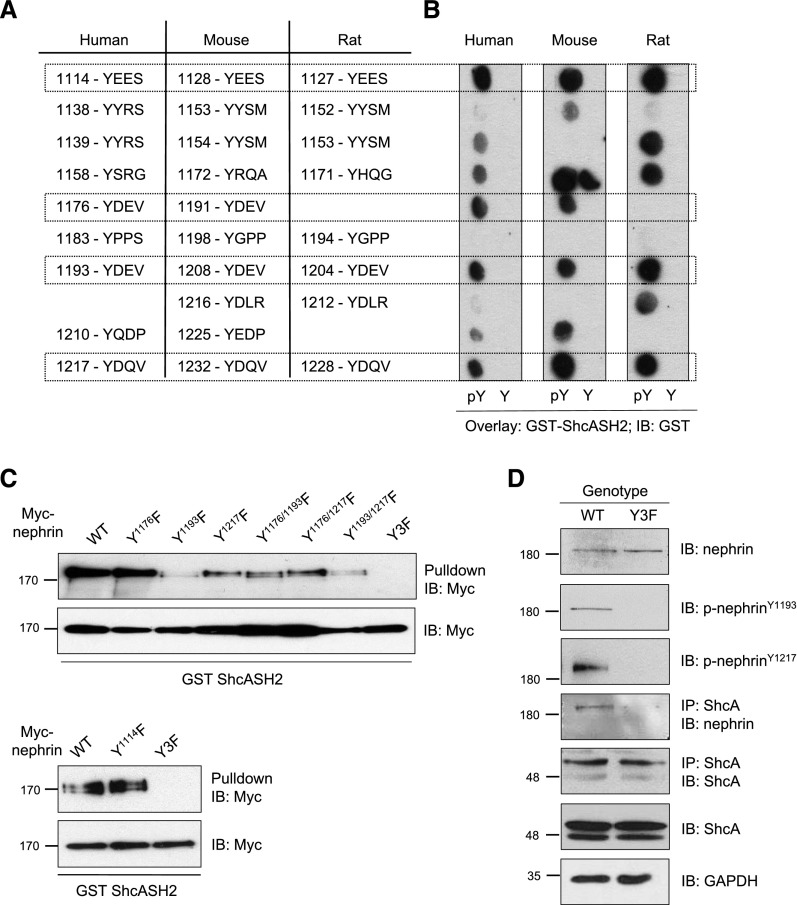
Figure 2.
ShcA binds multiple phosphotyrosine residues on nephrin in vitro and in vivo. (A) Conserved tyrosine residues in human, mouse, and rat nephrin. Boxes indicate residues demonstrated to be phosphorylated in vitro and/or in vivo. (B) Spot peptide arrays centered around tyrosine (Y) or phosphotyrosine (pY) of all phosphosites in human, mouse, and rat nephrin were incubated with purified GST-ShcA SH2 domain and immunoblotted with GST. Boxes indicate sites with positive anti-GST signal across all species. (C) Lysates from HEK293T cells overexpressing CA-Fyn with WT Myc-nephrin or variants with single, double, or triple mutations at tyrosine sites identified in (B) were incubated with GST-ShcA SH2 domain. Pulldown complexes immunoblotted for Myc indicate reduced binding of the ShcA SH2 domain to nephrin variants lacking Y1193, and a complete loss of binding with the Y3F variant. (D) Immunoblot (IB) for nephrin and ShcA in anti-ShcA immunoprecipitants (IP) from nephrinWT and nephrinY3F mouse glomerular lysates, confirming binding of ShcA and nephrin in vivo, and disruption of this interaction in mice lacking phosphorylated nephrin.