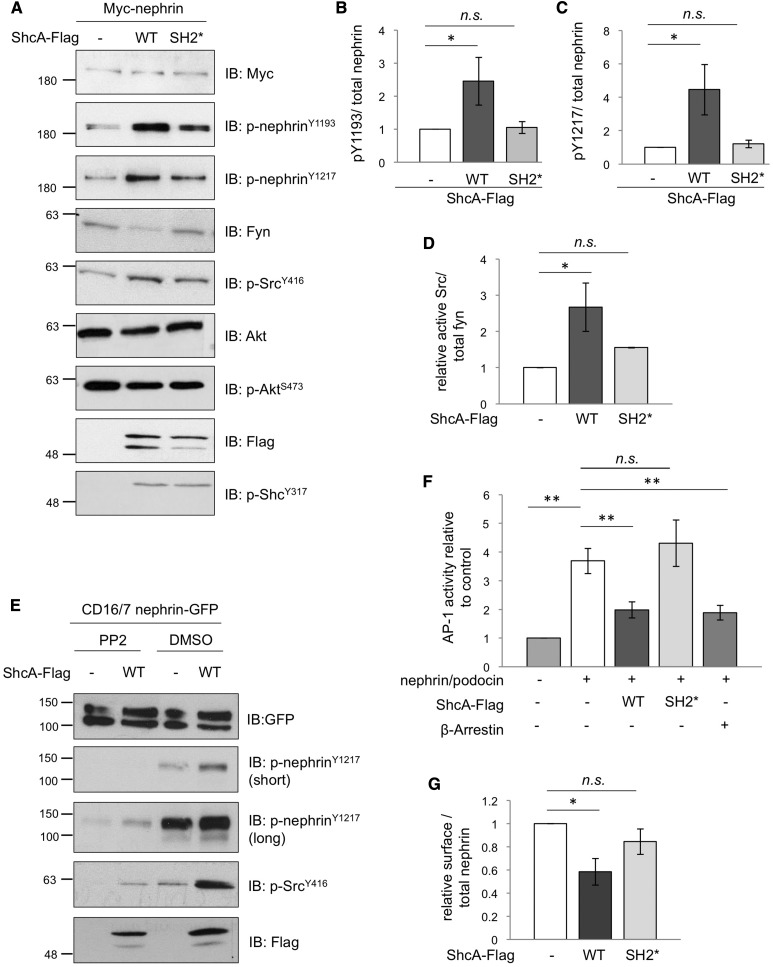
Figure 3.
ShcA promotes tyrosine phosphorylation and endocytosis of nephrin. (A) Lysates from HEK293T cells transiently coexpressing Myc-nephrin with WT p46/52 ShcA-Flag or an SH2* variant that cannot bind nephrin were immunoblotted (IB) as indicated. Changes in phosphorylation in nephrin and Src but not ShcA or Akt are detected. (B–D) Densitometric quantitation of p-nephrinY1193 (n=3), p-nephrinY1217 (n=3), and p-SrcY416 (n=4) from (A), respectively. (E) Lysates from HEK293T cells transiently coexpressing CD16/7-nephrin-GFP with or without ShcA-Flag were stimulated with anti-CD16 antibody in the presence or absence of the SFK inhibitor PP2 or vehicle alone (DMSO), and IB as indicated. PP2 suppresses the ShcA-induced increase in nephrin and Src phosphorylation. (F) HEK293T cells transiently coexpressing ShcA-Flag or β-arrestin with Myc-nephrin, podocin, AP1-firefly luciferase, and renilla luciferase were subject to a dual reporter assay. ShcA WT but not SH2* suppressed the nephrin/podocin-induced activation of AP-1, similar to β-arrestin (n=4). (G) HEK293T cells transiently coexpressing WT or SH2* ShcA-Flag with Myc-nephrin were subject to surface biotinylation. Densitometric comparison of streptavidin-precipitated biotinylated nephrin (surface) to total nephrin (input) indicates a reduction in surface nephrin with WT ShcA but not SH2* (n=3). All values were made relative to biotinylation result of cells transfected with Myc-nephrin alone. *P<0.05; **P<0.01, two-tailed t test.