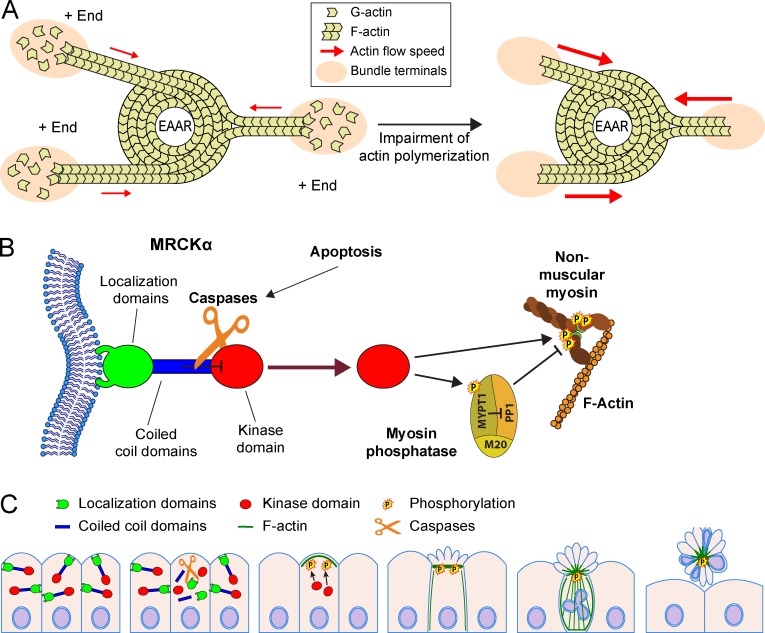
Figure 10.
MRCKα controls EAAR assembly and epithelial extrusion. (A) Cartoon showing the role of actin polymerization in EAAR mechanics. Actin is continuously assembled at the outermost bundle terminals (+ ends) coinciding with adhesion sites. The fast polymerizing activity occurring at the + ends is able to compensate for the actin flow generated by the centripetal pulling activity of EAAR, reducing the overall tension (red arrows) applied on bundles. Whenever actin polymerization activity is blocked, the uncompensated actin flow in bundles causes a rapid increase in bundle tension (red arrows) and a sudden increment of shortening rates of bundles, detachment of adhesions, and cell collapse. This model suggests that an efficient process of extrusion requires the coupling of myosin activity in the EAAR and down-modulation of actin polymerization at the bundles’ terminals. (B) MRCKα is a protein constituted by multiple domains that can be subclassified in three regions according to their functions: an N-terminal kinase domain (red), a central region composed of three coiled-coil domains (where CC2 and CC3 have inhibitory activity on the kinase domain; blue), and a C-terminal region including different domains deputed to binding to plasma membrane (green). Upon apoptosis activation, caspases cleave MRCKα in three parts corresponding to these three functional regions. The kinase domain is therefore relieved from the inhibitory activity exerted by CC2 and CC3 domains and is uncaged from the localization domains, acquiring solubility in the cytoplasm. The kinase domain, now free to reach and phosphorylate the regulatory small subunit of the nonmuscular myosin, promotes a massive contractile activity. This mechanism is further enforced by the phosphorylation of MYPT1 subunit of myosin phosphatase, preventing its inhibitory activity on myosin, and by the concomitant activation of other myosin kinases, such as ROCK1 and MLCK. (C) Caspase-mediated activation of MRCKα plays a crucial role during epithelial extrusion, by causing cortical contraction and EAAR assembly through the phosphorylation of the regulatory subunit of myosin. By generating centripetal forces, the EAAR causes shortening of actin bundles, collapse of cell body, and an upward movement of apoptotic cells toward the apical side of the epithelial layer. This sequence of events is required during epithelial extrusion, which is finally completed by basal ring constriction determined by the concerted action of neighboring cells.