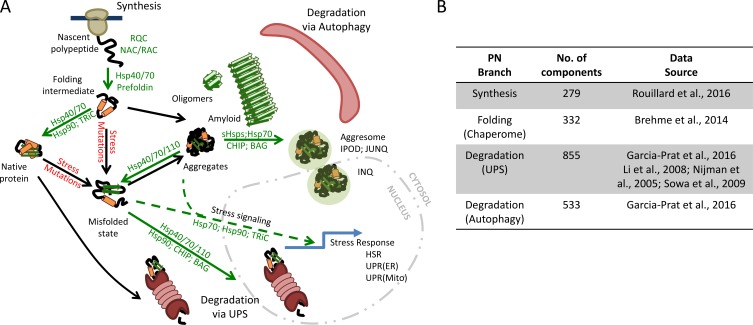
Figure 1.
The PN. (A) The flux of proteins through the PN relies on chaperones at many stages. PN capacity is sufficient to fold, often via intermediates, most newly synthesized polypeptides as they exit the ribosome. When a protein is no longer needed, it can be efficiently targeted for degradation either in the cytosol or nucleus via the UPS. Proteins that cannot be folded are also targeted for degradation via the UPS. During stress, the cell can increase PN capacity by activating a stress response. In aged or diseased cells, there is an increase in overall protein misfolding and aggregation, owing to increases in mutations and an overall decrease in PN capacity. These misfolded species may aggregate and/or be sequestered into large structures (aggresomes). A subset of misfolded species may form amyloid fibers that can further interfere with cellular processes. Chaperone factors that enhance particular steps are shown in green. Adapted from Hipp et al. (2014) and Kim et al. (2013). (B) Numbers for the major branches of the human PN, including synthesis, folding, and maintenance, and degradation branches, are shown. Datasets for generating these values were collected from the sources shown. The data were then arranged into nonoverlapping groups to represent the major PN branches. BAG, Bcl-2–associated athanogene; NAC, nascent chain-associated complex; RAC, ribosome-associated complex; RQC, ribosome quality control.