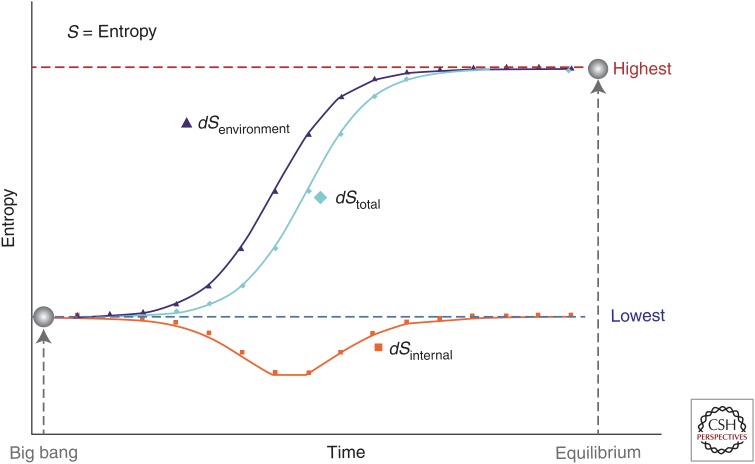
Figure 2.
Visualization of possible changes in entropy (dS) across time from the big bang (lowest entropy) to equilibrium (highest entropy). dS = deS + diS, where deS is the flow of entropy caused by exchanges with the surroundings (environment), and diS is the entropy production as a result of processes inside the system, such as diffusion, chemical reactions, and heat conduction (internal metabolism) (Prigogine 1978).