Figure 4.
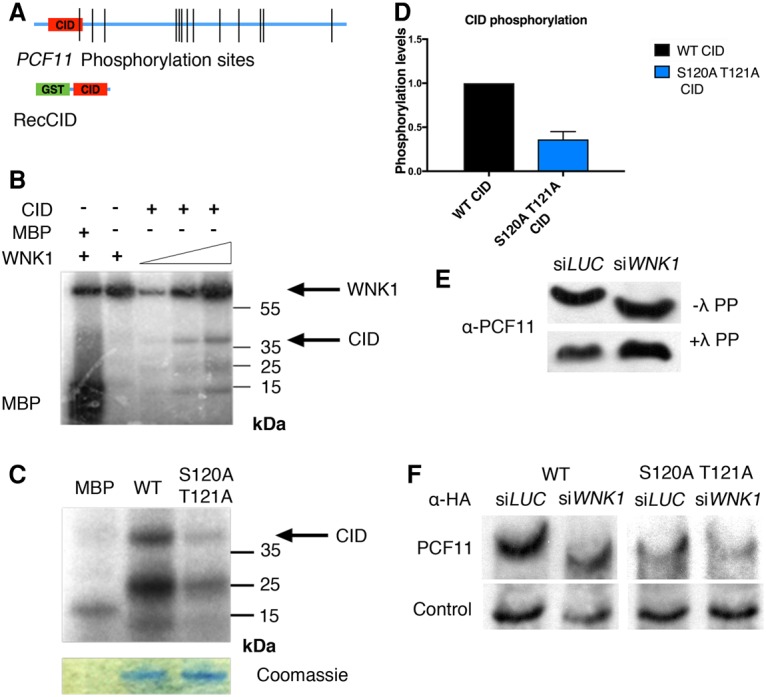
WNK1 phosphorylates the PCF11 CID. (A, top) Schematic representation of endogenous PCF11 phosphorylation sites identified in this study (Supplemental Table 2). (Bottom) Schematic representation of the recombinant GST-PCF11 CID. (B) Autoradiography of a recombinant WNK1 kinase domain in vitro kinase assay on the GST-PCF11 CID. MBP was used as a positive control for WNK1 phosphorylation. The upper band corresponds to autophosphorylated WNK1. The lower bands likely correspond to truncated products of the GST-PCF11 CID. (C) Autoradiography of a WNK1 in vitro kinase assay on the wild-type CID and S120A–T121A CID. Coomassie staining shows equal levels of GST-CID loading for wild type and the mutant. (D) Quantitation of kinase assays with the wild-type and S120A–T121A CID. Phosphorylation levels were measured in four independent repeats of the assay. Error bars represent SEM. (E) PhosTag Western blot for the detection of endogenous PCF11 migration upon WNK1 depletion. The same cell extracts treated with λ phosphatase were used as controls. (F) PhosTag Western blots comparing migration of wild-type PCF11-HA and S120A–T121A PCF11-HA in LUC and WNK1-depleted cells. The control lane shows the migration of an unspecific band recognized by the αHA antibody, which provides a positional and loading control.
