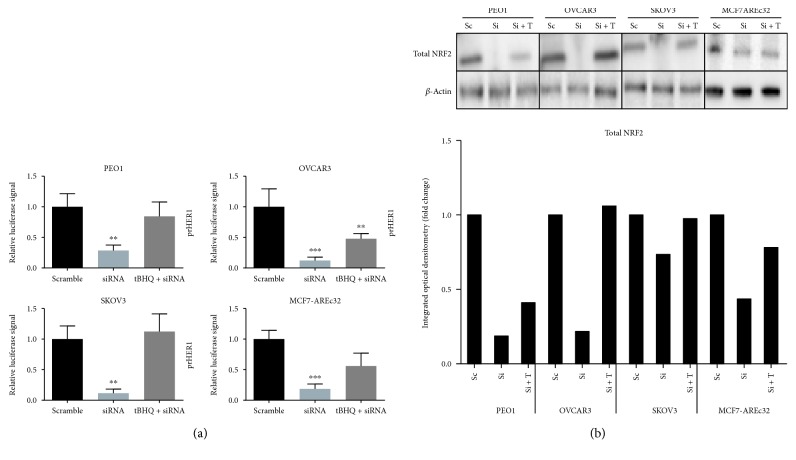
Figure 4.
Treatment with tBHQ reduces the knockdown effect of siRNA. (a) siRNA-mediated knockdown of NRF2 causes inhibition of its transcriptional antioxidant program and repression of HER1 level in both constitutive and tBHQ-induced states. MCF7-AREc32 which already contains stably cloned 8× cis-antioxidant response elements (ARE) driving NRF2-dependent expression of luciferase gene was left without any transfection while PEO1, OVCAR3, and SKOV3 cells were transfected with either empty PGL3 basic vector or 1 μg PGL3 basic vector with promoters of HER1-cloned driving HER1 expression of luciferase gene. Cotransfection with 0.2 μg pRL-CMV plasmid was performed as an internal transfection control. Where required, cotransfection with either scrambled RNA (Sc) or NRF2 siRNA was performed using 20 pmol siRNA. At 24 h after transfection, treatment with 100 μM tBHQ was performed where indicated for 4 h following which cells were processed for dual luciferase reporter assay (Promega) to record luciferase activity in multiplate reader (MODULUS, Promega). (b) Immunoblotting analysis showing repression of NRF2 following NRF2 knockdown by siRNA in PEO1, OVCAR3, and SKOV3 cell lines. Cells were either transfected with scrambled siRNA (Sc) or transfected with 75 pmol of NRF2 siRNA (Si). After 48 h, cells were either left untreated or treated with 100 μM tBHQ (T) for 4 h, before being processed for immunoblotting using relevant antibodies. β-Actin of the same blot was used as loading control. Bar chart shows NRF2 levels by quantifying immunoblot signal intensities obtained and expressed as fold change. Data in (a) are the means with ±S.D. of triplicates, normalised to scramble with statistical significance determined by one-way ANOVA followed by Tukey's post hoc test. ∗∗p < 0.01 and ∗∗∗p < 0.001.