Abstract
Background
Vitex negundo L. has been widely studied for its beneficial effect in inflammatory and pain conditions. The present study describes the isolation of two new bioactive chromone constituents from V. negundo and their in vivo evaluation for anti-inflammatory and antinociceptive activities.
Methods
Two new chromone derivatives, namely, methyl 3-(2-(5-hydroxy-6-methoxy-4-oxo-4H-chromen-2-yl)ethyl)benzoate (1) and 3-(1-hydroxy-2-(5-hydroxy-6-methoxy-4-oxo-4H-chromen-2-yl)ethyl)benzoic acid (2) were isolated from V. negundo and their structures were determined through various spectroscopic techniques including mass spectrometry, UV, IR, 1H NMR, 13C NMR, and two-dimensional-NMR like correlation spectroscopy and heteronuclear multiple bond correlation techniques. The isolated compounds (1–2) were tested for their prospective antinociceptive activity in acetic acid-induced abdominal constriction assay and anti-inflammatory activity in the carrageenan-induced paw edema assay in mice.
Results
Significant attenuation (P<0.001) of tonic visceral nociception was demonstrated by compound 1 and 2 at doses of 50 and 100 mg/kg. At similar doses, these compounds (1–2) also showed potent amelioration (P<0.001) of carrageenan-induced paw swelling.
Conclusion
The isolated chromone derivatives (1–2) from V. negundo are able to alleviate nociception and inflammation and the findings corroborated that V. negundo may be used as a potential source of antinociceptive and anti-inflammatory candidates.
Keywords: chromone derivatives, Vitex negundo, Verbenaceae, antinociceptive, anti-inflammatory
Introduction
Vitex negundo is a small tree belonging to family Verbenaceae and is native to Sri Lanka, India, Pakistan, Malaysia, China and East Africa.1 It grows in humid places in open forests and as crop in different parts of the world. Vitex negundo is of huge therapeutic importance and its leaves extract has been used as anti-inflammatory, analgesic and anti-itching agent in Ayurvedic tradition. Leaves extract of V. negundo are reported to have anti-hyperglycemic activity and hypouricemic activity as well.2,3
Vitex negundo has been used as a mosquito repellent agent and has good antiarthritic activity also. The seeds of V. negundo have antioxidant and anti-androgenic activities.1,4
Previous phytochemical investigation of V. negundo revealed the presence of terpenoids, lignans, flavonoids, alkaloids and glycosides as major chemical constituents.5 Similarly phenyl dihydronaphthalene-type lignan vitedoin A, a lignan alkaloid vitedoamine A and trinorlabdane type diterpene vitedoin B, 2α, 3α-dihroxyoleana-5, 12 –dien-28-oic acid, n-pentatriacontane have been reported from seeds of V. negundo,6,7 while viridiflorol, sabinene, casticin, negundin A, negundin B, vitrofolal A, various terpenes, flavanone and acids have been isolated from the leaves and roots of V. negundo.8–10 Further phytochemical investigation will provide information about the bioactive constituents present in V. negundo responsible for its therapeutic activity.
Pain and inflammation can result in serious consequences including severe symptoms, misery, stress and sometimes disabilities. Inflammation is a pervasive phenomenon that operates during severe perturbations of homeostasis, such as infection, injury, and exposure to contaminants, and is triggered by innate immune receptors that recognize pathogens and damaged cells.11 Inflammation is associated with many chronic human conditions and diseases, including allergy, atherosclerosis, cancer, arthritis and autoimmune diseases. Chromones exhibit putative anti-inflammatory properties12,13 and are considered as promising leads for anti-inflammatory drugs.14
The use of conventional drugs for the treatment of pain and inflammation has largely resulted in various side effects. These challenges have triggered scientific researchers all over the world in search of alternative therapy.15 Herbal medication may serve as a safe, effective and alternate treatment approach in the management of various diseases associated with pain and inflammation.16 Chromone and its analogs are considered as important pharmacophores, and privileged structures have been featured in a number of clinically used drugs.17
This present investigation describes the isolation and characterization of two new chromone derivatives from ethyl acetate fraction of V. negundo along with their anti-inflammatory and antinociceptive potential.
Methods
Extraction and isolation
The V. negundo plant (6.5 kg) was collected from Northern areas, KPK of Pakistan, and a voucher No 318b has been submitted in the herbarium of Botany Department of Government Post Graduate College, Bannu, Pakistan, where it was identified. The shade-dried plant was grinded into powder and extracted thrice with methanol. The crude extract (280 g) was obtained using a vacuum rotary evaporator, which was later partitioned into three fractions, namely, n-hexane (88 g), ethyl acetate (95 g) and n-butanol (80 g).
Column chromatography
The ethyl acetate fraction was selected for column chromatography on the basis of TLC using n-hexane as gradient of ethyl acetate to 100% followed by methanol. Ethyl acetate fraction was partitioned into various sub-fractions on the basis of polarity of compounds using silica gel chromatography with n-hexane: EtAcO (100:0–0:100) and sub-fraction 4–7 were subjected again to column chromatography and eluted with a ratio of 35:65 of ethyl acetate: hexane, while compound 2 (10 mg) was purified at polarity of 42:58 (Figure 1).
Figure 1.
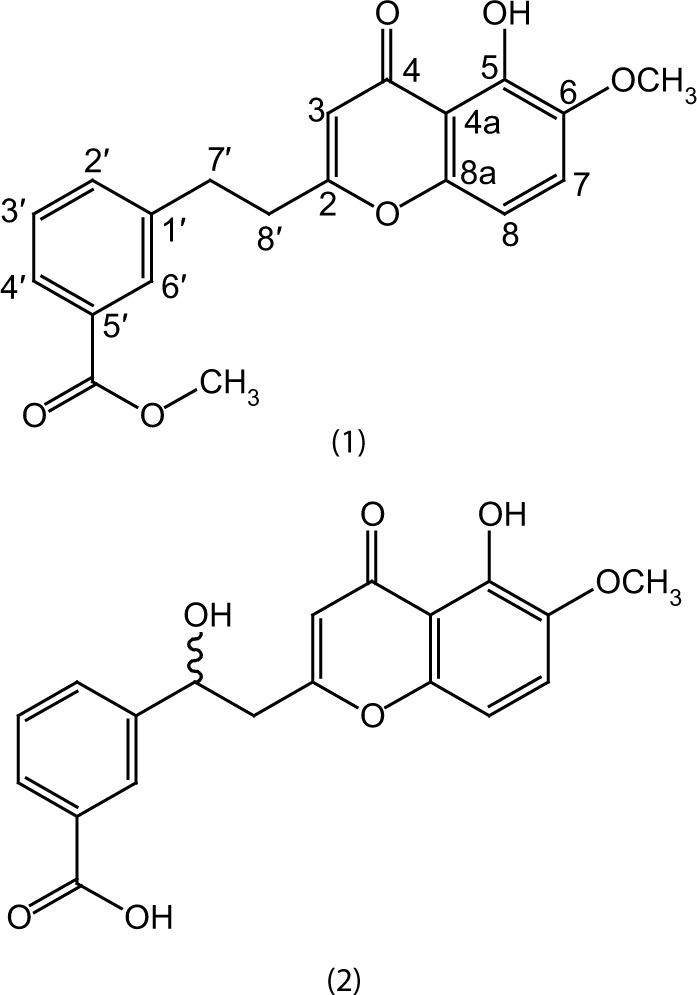
Structures of compound 1 and 2.
Experimental procedure
The IR spectra were recorded on JASCO-320-A spectrophotometer, and recording of UV spectra was done on UV-240. NMR spectra were recorded on Bruker AMX-500 spectrometer (500 MHz for 1H-NMR, 125 MHz for 13C-NMR) with tetramethylsilane as internal standard to record 1D-NMR and two-dimensional (2D)-NMR spectra with chemical shift values in ppm and coupling constant in hertz (Hz), while EI-MS and HR-EI-MS spectra were recorded on Varian MAT-312 spectrometer.
Characterization of compounds
Compound 1
Yellow solid: IR υmax (KBr): 1675, 1630, 1605, 1580 cm−1. UV λmax nm: 248 (3.2), 336 (4.3), 218 (5.1). Melting point: 204–206°C. EI-MS m/z: 354.1112 [M]+ (100), 322 (64), 308 (51), 272 (63), 254 (49), 244 (33). (Calculated 354.1103 for C20H18O6). 1H-NMR (500 MHz, CDCl3) δ ppm: 6.30 (1H, s), 12.10 (1H, s), 3.72 (3H, s), 7.54 (1H, d, J = 9.7 Hz), 7.10 (1H, d, J = 9.7 Hz), 7.80 (1H, d, J = 7.85 Hz), 7.54 (1H, t, J = 8.5 Hz), 7.94 (1H, d, J = 8.5 Hz), 8.04 (1H, brs), 3.29 (2H, m), 3.15 (2H, m). 13C-NMR (125 MHz, CDCl3) δ ppm: 166.7(C-2), 113.1(C-3), 180.6(C-4), 115.8(C-4a), 156.4 (C-5), 146.7 (C-6), 56.4 (6-OMe), 122.1 (C-7), 109.9 (C-8), 159.1 (C-8a), 142.3 (C-1′), 131.2 (C-2′), 130.8 (C-3′), 128.7 (C-4′), 139.3 (C-5′), 133.6 (C-6′), 37.1 (C-7′), 41.2 (C-8′), 170.4 (C-1′′), 54.2 (1′′-OMe).
Compound 2
Pale yellow gummy solid: IR υmax (KBr): 3340, 2990, 1660–1665, 1602 cm−1. UV λmax nm: 330 (4.7), 242 (5.3), 223 (5.9). Melting point: 174–175°C. EI-MS m/z: 356.0889[M]+ (100), 324 (74), 380 (64), 262 (69), 242 (35), 190 (42). (Calculated 356.0896 for C19H16O7). 1H-NMR (500 MHz, CDCl3) δ ppm: 6.20 (1H, s), 11.7 (1H, s), 3.80 (3H, s), 7.50 (1H, d, J = 10 Hz), 7.01 (1H, d, J = 10 Hz), 7.88 (1H, d, J = 8.2 Hz), 7.58 (1H, t, J = 8.1 Hz), 8.18 (1H, d, J = 8.1 Hz), 8.26 (1H, brs), 5.91 (1H, dd, J = 9.1, 5.4 Hz), 3.20 (1H, dd, J = 15.4, 5.4 Hz), 3.51 (1H, dd, J = 15.4, 9.1 Hz). 13C-NMR (125 MHz, CDCl3) δ ppm: 163.1 (C-2), 114.4 (C-3), 181.1 (C-4), 117.3 (C-4a), 154.3 (C-5), 149.1 (C-6), 58.4 (6-OMe), 125.1 (C-7), 110.3 (C-8), 156.1 (C-8a), 151.4 (C-1′), 131.4 (C-2′), 130.2 (C-3′), 129.4 (C-4′), 142.1 (C-5′), 132.6 (C-6′), 78.1 (C-7′), 49.2 (C-8′), 177.1 (C-1′′).
Animals
BALB/c mice of either sex weighing 25–35 g were purchased from the National Institute of Health (NIH), Islamabad, and were maintained in a 12-hour light/dark cycle at 22±2°C throughout the study. Animals were given ad libitum access to food and water. Experiments on animals were performed in compliance with the NIH guidelines for the care and use of laboratory animals. The experimental protocols were approved by the Ethical Committee of the Department of Pharmacy, University of Peshawar, Pakistan (registration number: 04/EC-15/Pharm).
Acetic acid-induced abdominal constriction assay
The peripheral antinociceptive activity of compound 1 and 2 was determined using the acetic acid-induced abdominal constriction assay.18 Animals weighing 25–28 g were divided into six groups (6 animals per group). Group I received normal saline. Group II was administered with the standard diclofenac sodium (50 mg/kg, intraperitoneal injection [ i.p.]). Group III and IV received compound 1 at doses of 50 and 100 mg/kg, while group V and VI were similarly treated with compound 2 at doses of 50 and 100 mg/kg, respectively, through an oral gavage tube. After 1 hour of treatment, the animals were injected with 1% acetic acid (10 ml/kg, i.p.). The number of writhes was counted after 10 minutes of acetic acid injection and the observation was continued for 20 minutes.
The percent protection against irritant-induced abdominal constriction was taken as index of a antinociception and was calculated by the following formula:
Carrageenan-induced paw edema assay
The anti-inflammatory activity of compound 1 and 2 was determined using the carrageenan-induced paw edema assay.19 Animals weighing 30–35 g were divided into seven groups (6 animals per group). Compound 1 and 2 were administered by an oral gavage tube in doses of 50 and 100 mg/kg. Diclofenac sodium was used as standard and was injected i.p. at a dose of 50 mg/kg. After 1 hour of treatment, the animals in groups II–VII were administered with 50 μL of 1% solution of car-rageenan, injected into the plantar surface of the left hind paw. The anti-inflammatory effect was evaluated by measuring the paw volume of each animal using a digital plethysmometer after each hour of the 3-hour study duration.
Statistical analysis
Data were expressed as mean ± SD or SEM. Statistical analysis was done by one-way analysis of variance followed by Dunnett’s or Tukey’s post hoc test using GraphPad Prism 5 (GraphPad Software Inc. San Diego CA, USA)
Results and discussion
Two new chromone derivatives (1–2) were isolated from ethyl acetate fraction of V. negundo through successive column chromatography and their characterization was carried out through different spectroscopic techniques.
Compound 1 was isolated as yellow solid form with molecular formula of C20H18O6, which was consistent with the molecular ion peak of [M]+ 354.1109 in HR-EI-MS. The absorption bands of UV at 248, 336 and 318 nm along with IR spectrum at 1675, 1630, 1605 and 1580 cm−1 suggested the presence of chromone ring in compound 1.
1H-NMR spectrum of compound 1 showed the presence of a hydroxyl group a with chemical shift value δH of 12.10 and two methoxyl group appeared at δH 3.72 (3H, s) and δH 3.60 (3H, s). Similarly, the two ortho-coupled protons with a chemical shift value of δH 7.10 and 7.54 (each having d, J = 9.7 Hz) were observed in 1H-NMR. The presence of three other aromatic protons appeared at δH 7.80 (d, J = 7.85 Hz), 7.45 (t, J = 8.5 Hz) and 7.94 (d, J = 8.5 Hz) and two multiplets at δH 3.29 and 3.15 were also revealed by 1H-NMR spectrum (Table 1).
Table 1.
1H-NMR (500 MHz) of compound 1 and 2 in CDCl3 (δH, J in Hz)
| Carbon no | Compound 1 1H-NMR (δH ppm) | Compound 2 1H-NMR (δH ppm) |
|---|---|---|
| 1 | – | – |
| 2 | – | – |
| 3 | 6.30 (1H, s) | 6.20 (1H, s) |
| 4 | – | – |
| 4a | – | – |
| 5-OH | 12.10 (1H, s) | 11.7 (1H, s) |
| 6-OMe | 3.72 (3H, s) | 3.80 (3H, s) |
| 7 | 7.54 (1H, d, J = 9.7 Hz) | 7.50 (1H, d, J = 10 Hz) |
| 8 | 7.10 (1H, d, J = 9.7 Hz) | 7.01 (1H, d, J = 10 Hz) |
| 8a | – | – |
| 1′ | – | – |
| 2′ | 7.80 (1H, d, J = 7.85 Hz) | 7.88 (1H, d, J = 8.2 Hz) |
| 3′ | 7.54 (1H, t, J = 8.5 Hz) | 7.58 (1H, t, J = 8.1 Hz) |
| 4′ | 7.94 (1H, d, J = 8.5 Hz) | 8.18 (1H, d, J = 8.1 Hz) |
| 5′ | – | – |
| 6′ | 8.04 (1H, brs) | 8.26 (1H, brs) |
| 7′ | 3.29 (2H, m) | 5.91 (1H, dd, J = 9.1, 5.4 Hz) |
| 8′ | 3.15 (2H, m) | 3.20 (1H, dd, J = 15.4, 5.4 Hz) |
| 3.51 (1H, dd, J = 15.4, 9.1 Hz) |
The 13C-NMR spectrum revealed the presence of two carbonyl carbons at δC 180.6 and 170.4 and two methoxy carbons at δC 54.2 and 56.4. Two methylene carbons with chemical shift values δC 41.2 and 37.1, while seven methine carbons with δC 122.1, 109.9, 113.1, 131.2, 130.8, 128.7 and 133.6 were also observed in the 13C-NMR spectrum (Table 2). The heteronuclear multiple bond correlation (HMBC) spectrum suggested the position of a hydroxyl group at position 5 of chromone ring as the hydroxyl proton (δH 12.10) showed correlation with carbon C-4a (δC 115.8) and C-6 (δC 146.7) and weak interaction with C-4, while the methoxyl protons (δH 3.72) showed HMBC cross-peaks with C-5 (δC 156.4), C-6 (δC 146.7) and C-7 (δC 122.1), thereby confirming the position of methoxyl group at position 6. The alkyl side chain was located at position 2 of chromone ring as suggested by the HMBC cross peaks of methylene protons (C-8′, δH 3.15) with C-2 (δC 166.7), C-3 (δC 113.1) and C-7′(δC 37.1) (Figure 2). The presence of a methyl ester at C-5′ was evident from the HMBC correlations as −OCH3 protons showed strong correlation with the carbonyl carbon (δC 170.4), which was directly connected with −OCH3 and C-5′ (δC 139.3). The methylene protons at H-7′ (δH 3.27) were connected with C-8′ (δC 41.2), C-1′ (δC 142.3), C-2′ (δC 131.2) and C-6′ (δC 133.6) through HMBC experiment. Similarly, the positions of aromatic protons were also justified by the HMBC correlations; protons at H-6′ (δH 8.04) showed cross-peak correlation with carbonyl carbon (δC 170.4) of ester located at C-5′ (δC 139.3) and C-1′ (δC 142.3), while H-4′ proton (δH 7.94) showed correlation with carbonyl carbon of ester placed at C-5′ in the HMBC experiment.
Table 2.
13C NMR (125 MHz) of compound 1 and 2 in CDCl3
| Carbon no | Compound 1 13C-NMR (δC ppm) | Compound 2 13C-NMR (δC ppm) |
|---|---|---|
| 1 | – | – |
| 2 | 166.7 | 163.1 |
| 3 | 113.1 | 114.4 |
| 4 | 180.6 | 181.1 |
| 4a | 115.8 | 117.3 |
| 5 | 156.4 | 154.3 |
| 6 | 146.7 | 149.1 |
| 6-OMe | 56.4 | 58.4 |
| 7 | 122.1 | 125.1 |
| 8 | 109.9 | 110.3 |
| 8a | 159.1 | 156.1 |
| 1′ | 142.3 | 151.4 |
| 2′ | 131.2 | 131.4 |
| 3′ | 130.8 | 130.2 |
| 4′ | 128.7 | 129.4 |
| 5′ | 139.3 | 142.1 |
| 6′ | 133.6 | 132.6 |
| 7′ | 37.1 | 78.1 |
| 8′ | 41.2 | 49.2 |
| 1′′ | 170.4 | 177.1 |
| 1′′-OMe | 54.2 | – |
Figure 2.
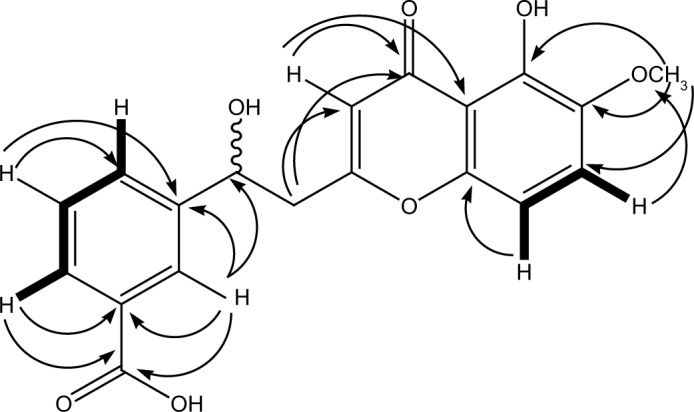
Important COSY (▬) and HMBC (→) correlations of compound 2.
Abbreviations: COSY, correlation spectroscopy; HMBC, heteronuclear multiple bond correlation.
Correlation spectroscopy (COSY) was quite supportive in the accurate placement of protons in the structure. COSY correlation was observed between H-2′/H-3′ and H-3′/ H-4′. All these spectral data and comparison with the literature20 suggested compound 1 as methyl 3-(2-(5-hydroxy-6-me-thoxy-4-oxo-4H-chromen-2-yl) ethyl) benzoate.
Compound 2 was obtained as a pale yellow gummy solid from the ethyl acetate fraction of V. negundo and was assigned a molecular formula of C19H16O7 on the basis of molecular ion peak at m/z [M]+ 356.0896 in the HR-EI-MS. The presence of chromone ring was suggested by the absorption bands of UV at 330, 242 and 223 nm in combination with IR spectral data as 3340, 2990 (for acid moiety) and 1660–1665, 1602 cm−1, which is quite similar to compound 1. All the 1H-NMR and 13C-NMR spectral data of compound 2 were similar to compound 1; the only difference observed was the presence of hydroxymethine in place of one methylene group of alkyl moiety (C-7′ and C-8′) (Tables 1 and 2). Similarly the presence of acid moiety at C-5′ was evident from IR, 1H-NMR, 13C-NMR supported by the mass spectrum. The 1H-NMR spectrum revealed chemical shift values for two methylene protons as δH 3.20 (dd, J = 15.4, 5.4 Hz, H-8′) and δH 3.51 (dd, J = 15.4, 9.1, H-8′), while proton of hydroxymethine group appeared at δH 5.91 (dd, J = 9.1, 5.4) for H-8′ and H-7′, respectively.
13C-NMR disclosed the presence of 19 carbons; the major signals in broad band were the carbonyl carbon of acidic moiety stretched downfield, which appeared at δc 177.1, while the methine signal at C-7′ resonated at δc 78.1 along with methylene group at C-8′ centered at δc 49.2. The quaternary carbon at position C-1′ appeared at δc 151.4.
The position of hydoxymethine group was confirmed at position C-7′ through HMBC spectrum data, as proton of hydroxymethine showed cross-peaks with C-1′, C-2′ and C-6′, C-8′ and C-2 (Figure 2). Similarly the aromatic protons H-3′, H-4′ and H-6′ showed strong correlations with the carbonyl carbon of acid group in the HMBC experiment. The final structure suggested for compound 2 on the basis of abovementioned spectral data was 3-(1-hydroxy-2-(5-hydroxy-6-methoxy-4-oxo-4H-chromen-2-yl)ethyl)benzoic acid.
Both the isolated chromone derivatives (1–2) were screened for potential antinociceptive activity in the acetic acid-induced abdominal constriction assay. During an observation period of 20 minutes (F5,30 =22.06, P<0.0001), antinociceptive effect (P<0.001) was produced by compound (1–2) at all the tested doses (50 and 100 mg/kg) as demonstrated by a reduction in the number of acetic acid-induced abdominal constrictions compared to the saline-treated group. Similarly, the standard analgesic diclofenac sodium also produced a robust analgesic effect as a significant amelioration (P<0.001) of nociception was observed at a dose of 50 mg/kg (Figure 3).
Figure 3.
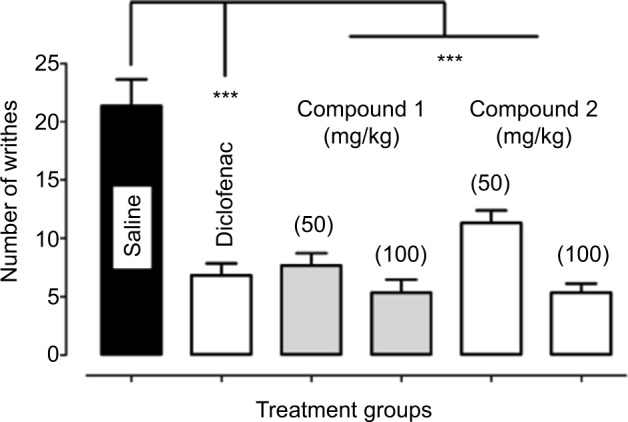
Antinociceptive activity of compound 1 and 2 in the acetic acid-induced abdominal constriction assay.
Notes: Values expressed as mean number of writhes ± SEM. One-way analysis of variance followed by Dunnett’s post hoc test. ***P<0.001 compared to saline treated group, n=6 mice per group.
Pain is a major health problem affecting patients’ quality of life and has significant impact on both the sufferers and the broader community, imparting high health costs and economic loss to the society.21 The acetic acid-induced nociceptive response results from the action of prostaglandins and other mediators by stimulating the sensory pathways in the mouse peritoneum that ultimately incite a viscero-somatic reflex manifested as abdominal writhing.22 The analgesic activity of test compound is inferred from a decrease in the frequency of abdominal writhing. According to literature, analgesics have profound effects on the acetic acid-induced nociception23 and a broad range of compounds have already been reported to depress the generation of nociceptive pain impulses.24,25 However, current strategies for the treatment of pain are inadequate and their effectiveness is limited by the extent of pain relief provided, occurrence of significant adverse effects or abuse liability. Chromones are a group of naturally occurring compounds reported to have antimicrobial activity, anti-allergic activity and muscular relaxation effects.26 In addition, chromones also exhibit potential analgesic effects by interacting with cyclooxygenases.14
The prospective anti-inflammatory activity of the isolated chromone derivatives was evaluated through the by well-known carrageenan-induced paw edema assay. As shown in Table 3, animals treated with carrageenan alone exhibited significant elevation (P<0.001) of paw volume at each hour of the study period. Treatment with compound 1 and 2 afforded a protective effect at doses of 50 and 100 mg/kg and resulted in a significant reduction (P<0.001) of the carrageenan-induced paw edema in the 1st hour (F6,35 =14.80, P<0.0001), 2nd hour (F6,35 =15.86, P<0.0001) and 3rd hour (F6,35 =15.25, P<0.0001) of study. Similarly, the standard diclofenac sodium also showed a higher anti-inflammatory effect by causing a significant reduction (P< 0.001) of paw edema at a dose of 50 mg/kg during the entire 3 hours of study duration.
Table 3.
Anti-inflammatory activity of compound 1 and 2 in carrageenan-induced paw edema assay
| Treatment | First hour | Second hour | Third hour |
|---|---|---|---|
| Group 1 | 0.225 ± 0.024 | 0.233 ± 0.024 | 0.243 ± 0.032 |
| Saline | |||
| Group II | 0.341 ± 0.029### | 0.373 ± 0.028### | 0.385 ± 0.018### |
| Carrageenan | |||
| Group III | 0.235 ± 0.024*** | 0.240 ± 0.023*** | 0.271 ± 0.027*** |
| Diclofenac (50 mg/kg) | |||
| Group IV | 0.228 ± 0.032*** | 0.245 ± 0.040*** | 0.266 ± 0.044*** |
| Compound 1 (50 mg/kg) | |||
| Group V | 0.233 ± 0.029*** | 0.235 ± 0.036*** | 0.256 ± 0.026*** |
| Compound 1 (100 mg/kg) | |||
| Group VI | 0.258 ± 0.018*** | 0.255 ± 0.032*** | 0.260 ± 0.030*** |
| Compound 2 (50 mg/kg) | |||
| Group VII | 0.263 ± 0.021*** | 0.248 ± 0.025*** | 0.275 ± 0.022*** |
| Compound 2 (100 mg/kg) |
Notes:
Values expressed as mean paw volume (mL) ± SD. Analysis of variance followed by Tukey’s post hoc test.
P<0.001 compared to group 1,
P<0.001 compared to group 2, n=6 mice per group.
Inflammation is a pervasive phenomenon that operates during severe perturbations of homeostasis, such as infection, injury, and exposure to contaminants, and is triggered by innate immune receptors that recognize pathogens and damaged cells.11 Inflammation is associated with many chronic human conditions and diseases, including allergy, atherosclerosis, cancer, arthritis and autoimmune diseases. Carrageenan has been used in cell-based and animal experiments to cause inflammation, primarily to study mediators of inflammation and anti-inflammatory therapeutics.27–29 Inflammation induced by carrageenan produces the cardinal features of inflammation and has been shown to mimic human clinical conditions.30,31 Natural products possess clinical effectiveness in the prevention and treatment of inflammatory conditions32 and their therapeutic prospective has been advocated to the marked anti-inflammatory properties in both in vivo animal models33,34 and in vitro human cells’35–37 inflammation paradigms. Chromones exhibit putative anti-inflammatory properties.12,13 and are considered as promising leads for anti-inflammatory drugs.14
Conclusion
Two new chromone derivatives methyl 3-(2-(5-hydroxy-6-methoxy-4-oxo-4H-chromen-2-yl)ethyl)benzoate and 3-(1-hydroxy-2-(5-hydroxy-6-methoxy-4-oxo-4H-chromen-2-yl)ethyl)benzoic acid were isolated from V. negundo through repeated column chromatography and their characterization was done through different spectroscopic techniques including IR, UV, 1D-NMR, 2D-NMR and mass spectrometry. The isolated chromone derivatives robustly ameliorated the irritant-induced nociceptive behavior and paw edema, therefore suggestive of analgesic and anti-inflammatory propensities. Further studies are warranted to investigate the molecular mechanisms involved in their beneficial potential to relieve pain and inflammation by utilizing both the in vivo animals and in vitro human cell models. These compounds may serve as an adjuvant therapy for the treatment of diseases involving these pathological conditions.
Acknowledgments
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this Prolific Research group (PRG-1437-29).
Footnotes
Author contributions
Ajmal Khan and Umar Farooq conceived and designed the experiments; Sadia Naz, Irfan Ullah and Muhammad Shahid performed the experiments; Abdur Rauf and Yahia Nasser Mabkhot analyzed the data; and Umar Farooq and Ajmal Khan wrote the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zheng CJ, Huang BK, Han T, et al. Nitric oxide scavenging lignans from Vitex negundo seeds. J Nat Prod. 2009;72(9):1627–1630. doi: 10.1021/np900320e. [DOI] [PubMed] [Google Scholar]
- 2.Dharmasiri MG, Jayakody JR, Galhena G, Liyanage SS, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87(2–3):199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 3.Umamaheswari M, AsokKumar K, Somasundaram A, Sivashanmugam T, Subhadradevi V, Ravi TK. Xanthine oxidase inhibitory activity of some Indian medical plants. J Ethnopharmacol. 2007;109(3):547–551. doi: 10.1016/j.jep.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res. 2003;17(2):129–134. doi: 10.1002/ptr.1088. [DOI] [PubMed] [Google Scholar]
- 5.Díaz F, Chávez D, Lee D, et al. Cytotoxic flavone analogues of vitexicarpin, a constituent of the leaves of Vitex negundo. J Nat Prod. 2003;66(6):865–867. doi: 10.1021/np0300784. [DOI] [PubMed] [Google Scholar]
- 6.Chawla AS, Sharma AK, Handa SS, Dhar KL. Chemical investigation and anti-inflammatory activity of Vitex negundo seeds. J Nat Prod. 1992;55(2):163–167. doi: 10.1021/np50080a002. [DOI] [PubMed] [Google Scholar]
- 7.Ono M, Nishida Y, Masuoka C, et al. Lignan derivatives and a nor-diterpene from the seeds of Vitex negundo. J Nat Prod. 2004;67(12):2073–2075. doi: 10.1021/np040102t. [DOI] [PubMed] [Google Scholar]
- 8.Khare CP. Encyclopedia of Indian Medicinal Plants. New York: Springer-Verlag Berlin Heidelberg; 2004. [Google Scholar]
- 9.Malik AA, Anis I, Khan SB, et al. Enzymes inhibiting lignans from Vitex negundo. Chem Pharm Bull. 2004;52(11):1269–1272. doi: 10.1248/cpb.52.1269. [DOI] [PubMed] [Google Scholar]
- 10.Singh V, Dayal R, Bartley JP. Volatile constituents of Vitex negundo leaves. Planta Med. 1999;65(6):580–582. doi: 10.1055/s-2006-960832. [DOI] [PubMed] [Google Scholar]
- 11.Ashley NT, Weil ZM, Nelson RJ. Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. [Google Scholar]
- 12.Liu H, Xu R, Feng L, et al. A novel chromone derivative with anti-inflammatory property via inhibition of ROS-dependent activation of TRAF6-ASK1-p38 pathway. PLoS One. 2012;7(8):e37168. doi: 10.1371/journal.pone.0037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzei M, Sottofattori E, Dondero R, Ibrahim M, Melloni E, Michetti M. N, N-dialkylaminosubstituted chromones and isoxazoles as potential anti-inflammatory agents. Farmaco. 1999;54(7):452–460. doi: 10.1016/s0014-827x(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 14.Shaveta, Singh A, Kaur M, Sharma S, Bhatti R, Singh P. Rational design, synthesis and evaluation of chromone-indole and chromone-pyrazole based conjugates: identification of a lead for anti-inflammatory drug. Eur J Med Chem. 2014;77:185–192. doi: 10.1016/j.ejmech.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Arome D, Sunday AI, Onalike EI, Amarachi A. Pain and inflammation: management by conventional and herbal therapy. Indian J Pain. 2014;28(1):5–12. [Google Scholar]
- 16.Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1:80. doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keri RS, Budagumpi S, Pai RK, Balakrishna RG. Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem. 2014;78:340–374. doi: 10.1016/j.ejmech.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003;225:115–121. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- 20.Yagura T, Ito M, Kiuchi F, Honda G, Shimada Y. Four new 2-(2-phenyl-ethyl) chromone derivatives from withered wood of Aquilaria sinensis. Chem Pharm Bull (Tokyo) 2003;51(5):560–564. doi: 10.1248/cpb.51.560. [DOI] [PubMed] [Google Scholar]
- 21.Hua S, Cabot PJ. Pain—novel targets and new technologies. Front Pharmacol. 2014;5:211. doi: 10.3389/fphar.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto H, Naraba H, Ueno A, et al. Induction of cyclooxygenase-2 causes an enhancement of writhing response in mice. Eur J Pharmacol. 1998;352(1):47–52. doi: 10.1016/s0014-2999(98)00340-9. [DOI] [PubMed] [Google Scholar]
- 23.Bentley GA, Newton SH, Starr J. Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. Br J Pharmacol. 1981;73(2):325–332. doi: 10.1111/j.1476-5381.1981.tb10425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray AM, Spencer PS, Sewell RD. The involvement of the opioidergic system in the antinociceptive mechanism of action of antidepressant compounds. Br J Pharmacol. 1998;124(4):669–674. doi: 10.1038/sj.bjp.0701882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray AM, Pache DM, Sewell RD. Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol. 1999;378(2):161–168. doi: 10.1016/s0014-2999(99)00464-1. [DOI] [PubMed] [Google Scholar]
- 26.Diwakar SD, Bhagwat SS, Shingare MS, Gill CH. Substituted 3-((Z)-2-(4-nitrophenyl)-2-(1H-tetrazol-5-yl) vinyl)-4H-chromen-4-ones as novel anti-MRSA agents: synthesis, SAR, and in-vitro assessment. Bioorg Med Chem Lett. 2008;18(16):4678–4681. doi: 10.1016/j.bmcl.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Fehrenbacher JC, Vasko MR, Duarte DB. Models of inflammation: carrageenan‐or complete Freund’s adjuvant (CFA)–induced edema and hypersensitivity in the rat. Curr Protoc Pharmacol. 2012 doi: 10.1002/0471141755.ph0504s56. Chapter:Unit5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris CJ. Carrageenan-induced paw edema in the rat and mouse. In: Winyard, Paul, Willoughby, Derek, editors. Inflammation Protocols. Bath, UK: Springer; 2003. pp. 115–121. [DOI] [PubMed] [Google Scholar]
- 29.Borthakur A, Bhattacharyya S, Anbazhagan AN, Kumar A, Dudeja PK, Tobacman JK. Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop. Biochim Biophys Acta. 2012;1822(8):1300–1307. doi: 10.1016/j.bbadis.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren K, Dubner R. Inflammatory models of pain and hyperalgesia. ILAR J. 1999;40(3):111–118. doi: 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- 31.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18(4):279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15(2):143–152. [PubMed] [Google Scholar]
- 33.Pandurangan A, Khosa RL, Hemalatha S. Anti-inflammatory activity of an alkaloid from Solanum trilobatum on acute and chronic inflammation models. Nat Prod Res. 2011;25(12):1132–1141. doi: 10.1080/14786410903370783. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez MA, Saenz MT, Garcia MD. Natural Products: anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J Pharm Pharmacol. 1998;50(10):1183–1186. doi: 10.1111/j.2042-7158.1998.tb03332.x. [DOI] [PubMed] [Google Scholar]
- 35.Shah M, Deshmukh SK, Verekar SA, et al. Anti-inflammatory properties of mutolide isolated from the fungus Lepidosphaeria species (PM0651419) Springerplus. 2015;4:706. doi: 10.1186/s40064-015-1493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 37.Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS. Curcumin prevents human dendritic cell response to immune stimulants. Biochem Biophys Res Commun. 2008;374(3):431–436. doi: 10.1016/j.bbrc.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]


