Abstract
The mammalian retina is an important model system for studying neural circuitry: Its role in sensation is clear, its cell types are relatively well defined, and its responses to natural stimuli—light patterns—can be studied in vitro. To solve the retina, we need to understand how the circuits pre-synaptic to its output neurons, ganglion cells, divide the visual scene into parallel representations to be assembled and interpreted by the brain. This requires identifying the component interneurons and understanding how their intrinsic properties and synapses generate circuit behaviors. Because the cellular composition and fundamental properties of the retina are shared across species, basic mechanisms studied in the genetically modifiable mouse retina apply to primate vision. We propose that the apparent complexity of retinal computation derives from a straightforward mechanism—a dynamic balance of synaptic excitation and inhibition regulated by use-dependent synaptic depression—applied differentially to the parallel pathways that feed ganglion cells.
Keywords: parallel pathways, bipolar cell, amacrine cell, ganglion cell, adaptation, synaptic depression
INTRODUCTION
Vision begins when light enters the eye through the pupil and is focused by the cornea and lens onto rod and cone photoreceptors. Photoreceptors convert light energy into changes in membrane potential that modulate tonic synaptic transmission to second-order neurons. These second-order neurons are organized into multiple circuits that operate in parallel and converge onto ganglion cells. Ganglion cell axons form the optic nerve, which branches so that axons of individual cell types selectively innervate specific central targets dedicated to a range of functions: orienting attention, regulating circadian rhythms, controlling eye movements, and of course, generating visual perception (Dhande et al. 2015). Because each type of ganglion cell receives input from a unique combination of parallel interneuron pathways, each type responds differently to the same visual stimulus (Roska & Werblin 2001, Briggman & Euler 2011). These unique responses represent the fundamental building blocks for the early stages of visual processing in the brain.
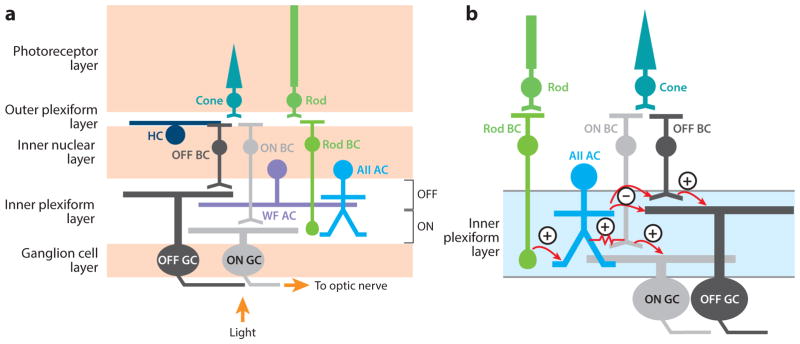
Mammalian retinal circuits are constructed from ~100 specific cell types (Masland 2012, Euler et al. 2014, Seung & Sümbül 2014, Sanes & Masland 2015). These include 3–4 types of photoreceptor (1 rod and 2–3 cones), 50–70 types of interneuron (horizontal, bipolar, and amacrine cells), and 20–30 types of ganglion cells (Figure 1a). The numerical ranges reflect uncertainty in the identity of every cell type, each of which should have a unique combination of anatomical and physiological properties governed by protein expression (Huberman et al. 2008, Kim et al. 2008, Duan et al. 2014, Seung & Sümbül 2014). Cells of each type appear to tile the retina, such that all regions are covered by at least one member of that type, with varying degrees of overlap (Borghuis et al. 2008, Wässle et al. 2009, Field et al. 2010, Masland 2012). Cell size varies within the retina: Neurons with smaller dendritic fields are found in areas devoted to high-acuity vision, such as the primate fovea (Kolb & Marshak 2003). In the mouse retina, different cell types have their smallest members in either the inferior retina, for viewing the sky, or in the dorsal/temporal retina, for binocular vision (Zhang et al. 2012, Bleckert et al. 2014) (Figure 2).
Figure 1.
Retinal circuits. (a) The cellular and synaptic (i.e., plexiform) layers of the retina. Some of the various cell types composing the five classes of neurons are shown: rod and cone photoreceptors, horizontal cells (HCs), ON and OFF cone bipolar cells (BCs), rod BCs, AII and wide-field (WF) amacrine cells (ACs), and ON and OFF ganglion cells (GCs). The ON and OFF BC axon terminals and GC dendrites stratify in separate halves of the inner plexiform layer. (b) Several cell types from panel a, redrawn to illustrate how rod signals pass through the inner retina. Excitatory (+) and inhibitory (−) synapses are shown. A gap junction (denoted by the resistor symbol) allows bidirectional current flow between AII ACs and ON cone BCs. The AII AC splits the ON rod BC signal into ON and OFF components using either electrical (gap junction, ON) or chemical (glycinergic, OFF) synapses. Note that in daylight conditions, cone-mediated drive to the AII influences the OFF pathway as follows: cone → ON cone BC→ AII AC→ OFF BC and GC.
Figure 2.
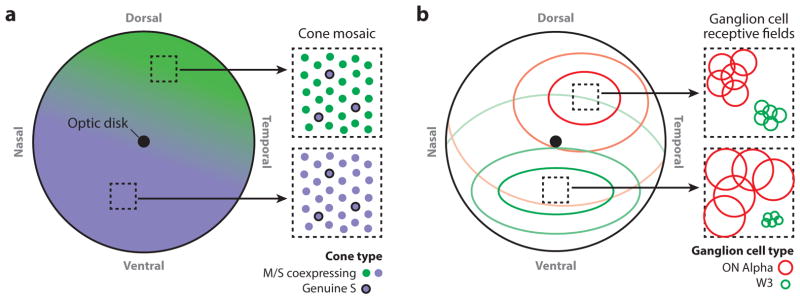
Regional variation in cell density across the retina. (a) Map depicting the gradient of cone opsin coexpression across the mouse retina. In the dorsal/temporal retina, the coexpressing cones, which represent ~90–95% of the cone population, express primarily M opsin, which is most sensitive to green light. In the ventral/nasal retina, the co-expressing cones express primarily S opsin, which is most sensitive to UV light. In between these two extremes, there is a gradient of relative M/S opsin expression. Throughout the retina, approximately 5–10% of cones are genuine S cones and express exclusively S opsin. (b) Map depicting the regional differences in the size and density of two ganglion cell types. In the ventral retina, the W3 ganglion cell type is relatively small, and its mosaic is dense. In the dorsal/temporal retina, the ON αganglion cell type is relatively small, and its mosaic is dense. The contour lines indicate the relative density of either W3 ( green) or ON α(red ) ganglion cell receptive field centers, which is comparable to the dendritic field. The insets (right) show the changes in receptive field size and density for each cell type, illustrating five examples of each cell type within each region.
Our main thesis is that the apparent complexity of retinal computation (Gollisch & Meister 2010) derives from a straightforward mechanism—a dynamic balance of synaptic excitation and inhibition regulated by use-dependent synaptic depression—applied differentially to the various parallel pathways that feed ganglion cells. Thus, diverse retinal outputs encoded in the various ganglion cell types are variations on a simple theme. Here, we review recent functional and anatomical studies of retinal circuitry as they relate to parallel processing and circuit-level gain control, or adaptation. We focus on the mouse retina, for which accessible genetic manipulation has spurred a burst of experimental creativity, but we highlight mechanisms common to all mammals—indeed, to all vertebrates—where appropriate.
Some research topics are not reviewed here, including mechanisms of retinal development and the intrinsically photosensitive ganglion cells that express the photopigment melanopsin and modulate circadian rhythms and other functions. Rather, the reader is referred to excellent, recent reviews of these topics (Blankenship & Feller 2010, Okawa et al. 2014, Do & Yau 2010, LeGates et al. 2014).
PARALLEL EXCITATORY PATHWAYS ARE ESTABLISHED AT THE FIRST RETINAL SYNAPSES
Intensity and Spectral Differences Are Encoded by Photoreceptors
Vertebrate retinas use two types of conventional photoreceptors, rods and cones, both of which hyperpolarize to light. Rods, which can encode the absorption of single photons and are therefore far more sensitive than cones (Baylor 1996), are active during night vision, whereas cones are active during daylight vision. There is also a range of intermediate lighting conditions (e.g., twilight or daybreak) in which both rods and cones are active (Yin et al. 2006). In most mammalian retinas, including those of humans, rods outnumber cones by 20- or 30-to-1. Retinal pathways, however, are organized around the cones and their postsynaptic bipolar cells, which are older evolutionarily (Lamb 2009).
Each cone typically contains one of three photopigments (opsins + chromophore) with varying spectral sensitivities. In humans, most cones (~95%) show peak sensitivity to either middle (M, ~530 nm) or long (L, ~560) wavelengths. A few cones (~5%) show peak sensitivity to short wavelengths (S, ~430 nm) (Williams 2011). Most mammals, other than primates, have only two cone types: M and S. Cones in the mouse retina, however, differ from those in other mammalian retinas. Given our focus here on the mouse retina as an experimental model, we briefly consider these differences.
Mouse cones comprise two types: genuine S cones and coexpressing cones that express both M and S opsins (Nikonov et al. 2006, Baden et al. 2013). Coexpression occurs in a gradient across the retina: The M/S ratio is highest in the coexpressing cones of the superior retina and becomes almost purely S in the inferior retina (Applebury et al. 2000, Wang et al. 2011, Baden et al. 2013) (Figure 2a). The absorbance of mouse S opsin is shifted into the UV range (peak, 360 nm; Jacobs et al. 1991), and correspondingly, the mouse lens transmits UV light efficiently ( Jacobs & Williams 2007). Hence, a large fraction of the mouse retina contains cones with almost pure UV sensitivity, and across the retina, the S:M opsin ratio is ~3 or 4:1 (Applebury et al. 2000). Thus, mouse cones on average are not particularly sensitive to visible light. This likely explains why early studies of the mouse retina—which used visible light stimuli—showed that presumed cone-driven responses recorded from ganglion cells are slow: The authors likely stimulated rods primarily (see Wang et al. 2011). When UV light is used as a stimulus, the responses of mouse ganglion cells are faster, resembling those of primate ganglion cells to visible light (Wang et al. 2011, Borghuis et al. 2014).
Photoreceptors Feed Parallel Bipolar Cell Pathways
Photoreceptors make synapses onto about 12 types of bipolar cells, and it is at these synapses that photoresponses split into parallel signaling streams (Euler et al. 2014). In mouse, it has been possible to identify most bipolar cell types on the basis of protein expression, using either immunohistochemistry or transgenic expression of proteins such as eGFP (Wässle et al. 2009). Twelve bipolar cell types were identified in this way; a thirteenth was resolved by cluster analysis based on electron microscopy (EM) reconstructions (Helmstaedter et al. 2013). Each bipolar cell type collects inputs from 5–10 cones (Wässle et al. 2009). One bipolar type is connected exclusively to genuine S cones (Haverkamp et al. 2005), similar to a type observed in primate and ground squirrel retinas (Kouyama & Marshak 1992, Li & DeVries 2006). The others receive input from both genuine S cones and M/S opsin coexpressing cones or from M/S opsin coexpressing cones alone (Euler et al. 2014). An additional bipolar cell type—the rod bipolar cell—receives input from rods alone (Demb & Singer 2012). Similar numbers and types of cells have been found in other vertebrate retinas, including the rabbit (Masland 2012), primate (Boycott & Wässle 1991, Puthussery et al. 2014), zebrafish (Connaughton et al. 2004), and salamander (Zhang & Wu 2009).
Cone bipolar cells release glutamate directly onto ganglion cells. The rod bipolar cell instead signals to ganglion cells indirectly via a specialized interneuron called the AII amacrine cell. The AII amacrine cell makes either excitatory (electrical) or inhibitory (glycinergic) synapses with different types of cone bipolar cells and ganglion cells; the circuitry of this unique pathway has been reviewed elsewhere (Demb & Singer 2012) and is depicted in Figure 1b.
Bipolar Cells Are Divided into ON and OFF Classes
The photoreceptor signal is first divided into ON and OFF pathways, which encode increments and decrements, respectively, in light intensity. Increments depolarize ON bipolar cells and hyperpolarize OFF bipolar cells (and vice versa) by well-understood synaptic mechanisms (Euler et al. 2014). Photoreceptors release glutamate, which acts on metabotropic receptors on ON cells and ionotropic receptors on OFF cells. The metabotropic receptors (mGluR6) are linked through a signaling cascade to a cation channel (TRPM1) such that receptor activation suppresses a cation conductance (Morgans et al. 2009). Thus, the ON response represents the reversal of this suppression. The ionotropic receptors of OFF bipolar cells are ligand-gated cation channels that directly gate an excitatory conductance (DeVries 2000, Borghuis et al. 2014, Puthussery et al. 2014). Thus, photoreceptor–ON bipolar cell synapses are sign reversing because light hyperpolarizes photoreceptors but depolarizes ON bipolar cells; photoreceptor–OFF bipolar cell synapses are sign conserving because light hyperpolarizes both cell types.
ON and OFF cone bipolar cell terminals contact the dendrites of their postsynaptic partners in the inner plexiform layer (IPL). Generally, ON and OFF synapses (axon terminals and postsynaptic processes) are localized to different halves of the IPL (Figure 1a). Thus, ON and OFF bipolar cells are identified anatomically by the localization of their axon terminals, and ON and OFF amacrine and ganglion cells are identified anatomically by the positions of their dendrites. There are at least two exceptions to this rule, though: Both dopaminergic amacrine cells and a type of intrinsically photosensitive ganglion cell (M1 melanopsin-expressing cells) receive en passant synapses from ON cone bipolar cell axons and therefore exhibit ON responses to photoreceptor stimulation despite having anatomical characteristics of OFF cells (Dumitrescu et al. 2009, Hoshi et al. 2009).
Temporal Processing in Parallel Bipolar Cell Pathways
Classically, ganglion cells were divided into distinct temporal channels that were broadly characterized as transient and sustained (Cleland et al. 1971). Transient cells encode changes in light intensity around a mean, whereas sustained cells encode the mean light level. It is now apparent that ganglion cell responses are partly inherited from their presynaptic circuits and that bipolar cells can be classified as transient or sustained (Roska & Werblin 2001). The temporal properties of bipolar cell outputs recently have been characterized using optical methods. In the mouse retina, fluorescent Ca2+ indicator imaging revealed transient signals in the terminals of bipolar cells (both ON and OFF types) that stratified near the middle of the IPL; more sustained signals were observed in bipolar cell terminals located near the nuclear boundaries (Baden et al. 2013). Calcium imaging is relatively slow, however, and so responses at high temporal frequencies were not resolved by this study. A faster imaging technique uses a genetically encoded biosensor for glutamate, iGluSnFR (Marvin et al. 2013), delivered to the retina using adeno-associated virus and broadly expressed in amacrine and ganglion cell processes postsynaptic to bipolar cell axon terminals. The iGluSnFR response more closely followed measurements of ganglion cell excitatory currents and could be modulated at ~8 Hz (Borghuis et al. 2014). iGluSnFR imaging demonstrated that transient glutamate release is localized to the most central layers of the IPL (Borghuis et al. 2013). Diversity in temporal channels between bipolar synapses also has been demonstrated using optical imaging in the fish retina (Odermatt et al. 2012). Interestingly, a recent study of salamander retina suggests that a single bipolar cell might provide temporally distinct outputs to different ganglion cell types (Asari & Meister 2012).
Mechanisms endowing the synaptic outputs of different bipolar cell types with distinct temporal responses include (a) postsynaptic glutamate receptors at photoreceptor–bipolar cell synapses, (b) intrinsic (e.g., voltage-sensitive) conductances, (c) dynamics of synaptic transmission from bipolar cell terminals, and (d ) inhibitory feedback and feed-forward circuits. Each of these is considered below.
Postsynaptic glutamate receptors
All ON bipolar cells express mGluR6, and the functional division into transient and sustained types might depend in part on differential expression of modulatory proteins in the associated second messenger pathway; e.g., alpha subunit GTPase activity (Martemyanov 2014). For OFF bipolar cells, studies of the ground squirrel retina suggested that the expression of AMPA receptors (AMPARs) or kainate receptors (KARs) in different bipolar cell types would generate transient or sustained responses, respectively (DeVries 2000), but recent work indicates that this mechanism is not universal. Both mouse and primate OFF bipolar cells express postsynaptic KARs exclusively, which indicates that temporal processing may originate through different combinations of KAR subunits and/or through mechanisms downstream of the postsynaptic conductance (Borghuis et al. 2014, Lindstrom et al. 2014, Puthussery et al. 2014).
Intrinsic conductances
Individual bipolar cell types express specific combinations of voltage-gated channels, which endow them with unique voltage responses to synaptic input. A recent study revealed distinct postsynaptic temporal processing characteristics of different ON bipolar cell types (Ichinose et al. 2014). Experiments combining light responses and current injection suggested that low-pass characteristics (i.e., suppression of high temporal frequencies) depended on intrinsic properties of the bipolar cells, whereas high-pass characteristics (i.e., suppression of low temporal frequencies) were generated through synaptic mechanisms. Electrophysiological and immunohistochemical analyses have demonstrated the expression of transient, voltage-gated conductances such as Ih, INa, and T-type Ca currents in different ON and OFF bipolar cells (de la Villa et al. 1998, DeVries et al. 2006, Cui & Pan 2008, Hu et al. 2009); and some bipolar cell types generate Na or Ca channel–dependent spikes in response to photoreceptor input, which should make the output more transient (Protti et al. 2000, Ichinose et al. 2005, Baden et al. 2011, Saszik & DeVries 2012). Thus, the postsynaptic responses of bipolar cells in the various parallel pathways are diverse and contribute to differential signaling by distinct pathways.
Transmission at bipolar cell synapses
In response to sustained presynaptic depolarization, release from the axon terminals of bipolar cells can be transient (von Gersdorff et al. 1998, Singer & Diamond 2003). The presynaptic active zones of a bipolar cell contain small, readily releasable vesicle pools that are depleted rapidly (Mennerick & Matthews 1996, Singer & Diamond 2006, Zhou et al. 2006, Oltedal & Hartveit 2010, Jarsky et al. 2011, Oesch & Diamond 2011). Thus, factors apart from the intrinsic dynamics of transmission (e.g., voltage-gated conductances and presynaptic inhibition) that control the time course of presynaptic depolarization likely play a significant role in creating the diversity of bipolar cell outputs. A caveat, however, is that current understanding of bipolar cell synapse function comes from detailed studies of only two model synapses: the rod bipolar cell synapse of the rodent retina and the Mb1 (mixed rod–cone bipolar cell 1) synapse of the teleost retina.
Feedback and feedforward inhibition
All bipolar cell terminals receive significant inhibitory input; inhibitory input is both reciprocal (i.e., feedback) and nonreciprocal (i.e., feedforward; Figure 3a) and operates to tune the outputs of parallel bipolar cell pathways on fine and coarse spatial scales (Werblin 2010, Eggers & Lukasiewicz 2011). A particularly interesting function of presynaptic inhibition is to modulate the outputs of OFF bipolar cells independently of direct photoreceptor input. Both transient and sustained release from OFF bipolar cells, as measured by iGluSnFR fluorescence on postsynaptic membranes or postsynaptic currents, could be modulated by presynaptic inhibitory synapses in the absence of functioning cone–OFF bipolar cell synapses (Borghuis et al. 2014). The apparent mechanism is cross-over inhibition, a form of synaptic modulation by inhibitory ON amacrine cells (Werblin 2010) (Figure 3a). These ON amacrine cells are depolarized by ON bipolar cells at light onset; therefore, OFF bipolar cells receive ON inhibition and hyperpolarize. At light offset, this inhibition is relieved (i.e., there is disinhibition) and the OFF bipolar cells depolarize. Synaptic outputs with different temporal features could be generated by inhibitory inputs with different time courses and/or by hyperpolarization-activated depolarizing conductances such as Ih and/or T-type Ca currents with varying kinetics. This finding indicates that crossover inhibition and direct cone input are complementary: Both depolarize the OFF bipolar cell to light decrements. This type of crossover inhibition, however, was not apparent in OFF pathway circuits in the primate retina (Puthussery et al. 2014), and therefore, it apparently differs between species.
Figure 3.
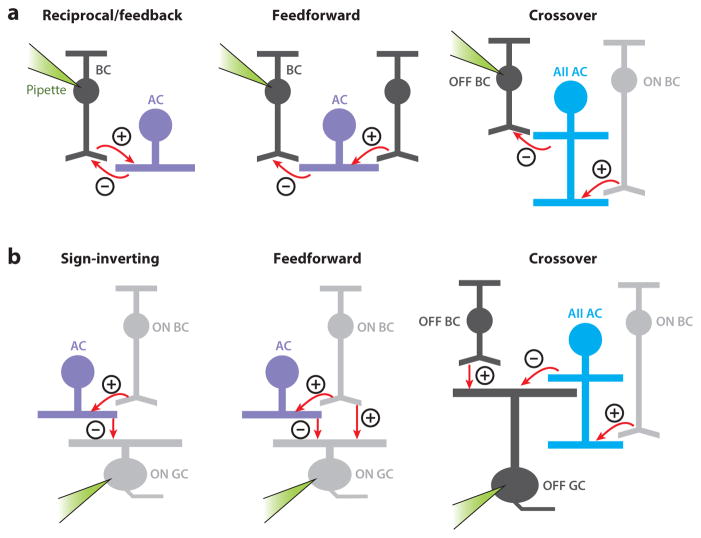
Synaptic motifs. (a) From the perspective of a bipolar cell (pipette attached), inhibition arising from amacrine cells (ACs) occurs via multiple synaptic motifs. Excitatory (+) and inhibitory (−) synapses are indicated; feedback and feedforward synapses can occur in both ON and OFF systems, and crossover inhibition acts between ON and OFF systems. The illustrated circuit is an ON → OFF inhibitory one, but the opposite pattern (OFF → ON) could also occur. (b) From the perspective of a ganglion cell (GC) (pipette attached), inhibition from ACs occurs via multiple synaptic motifs. This panel follows the same conventions as used in panel a.
Ganglion Cells Integrate the Outputs of Parallel Pathways
The synaptic outputs of different bipolar cell types are distributed to different ganglion cell types such that each ganglion cell receives input from at least one but typically multiple bipolar cell types with various numbers of synapses, or weights; likewise, each bipolar cell type provides a weighted input to multiple amacrine and ganglion cell types (Masland 2012, Dunn & Wong 2014). Thus, matching the weights from each bipolar cell type to the amacrine and ganglion cells is, in very general terms, the key problem in solving the retinal wiring diagram or connectome. Some of the bipolar–ganglion cell connections are known. Classic studies using serial EM reconstruction established the bipolar cell inputs to cat α and β ganglion cells (Freed & Sterling 1988, Cohen & Sterling 1992, Kolb & Nelson 1993). Confocal analysis suggested that mouse ON α ganglion cells receive a majority of their excitatory input from type 6 bipolar cells and a minority of input from type 7 bipolar cells (Schwartz et al. 2012). Optogenetic stimulation, using channelrhodopsin 2 expressed in specific bipolar cell types, showed that mouse direction-selective (DS) ganglion cells receive input from types 2 and 5 bipolar cells (Duan et al. 2014). A summary of proposed inputs to multiple mouse ganglion cell types, based on an EM data set (Helmstaedter et al. 2013), was presented recently (Dunn & Wong 2014).
The timing of transmission at bipolar cell–ganglion cell synapses also could be tuned by post-synaptic receptors. At excitatory synapses onto projection neurons elsewhere in the central nervous system, the coexpression of fast AMPA-type and slower Ca2+-permeable NMDA-type glutamate receptors plays a major role in determining the time course of synaptic excitation. In the retina, the distinct roles of these receptor types are less clear. Some ganglion cell types use NMDA receptors for the primary depolarizing response, whereas others have minimal NMDA receptor activity under physiological conditions (i.e., light-evoked responses with intact inhibition) (Manookin et al. 2010, Venkataramani & Taylor 2010, Buldyrev et al. 2012, Crook et al. 2014). Both receptor types can respond to rapidly modulated presynaptic glutamate release (Stafford et al. 2014). Beyond encoding light-evoked glutamate release, NMDA receptors could play a role in regulating the density and subtype of synaptic AMPA receptors ( Jones et al. 2012).
Nonlinear Subunits
Many bipolar cells converge onto a single ganglion cell, and the receptive field of each bipolar cell can be considered a small subunit of the larger receptive field of the ganglion cell (Figure 4). Early descriptions of the most common (and therefore smallest) ganglion cells (X/β cells in cat retinas and midget/parvocellular cells in primate retinas) suggested that ganglion cells integrated these subunits linearly (Enroth-Cugell & Robson 1966, Kaplan & Benardete 2001). It is apparent, however, that most ganglion cell types integrate their inputs nonlinearly (Schwartz & Rieke 2011). The classic example is the Y/α cell of the cat retina, which has a characteristic response to a stimulus composed of alternating thin black and white bars (i.e., a high–spatial frequency grating). When the temporal contrast of the high–spatial frequency grating was modulated sinusoidally, Y cells responded at twice the stimulus frequency (Enroth-Cugell & Robson 1966, Hochstein & Shapley 1976). This nonlinear response could be explained by the population behavior of the bipolar cell subunits: In the first phase, half of the subunits were exposed to bright bars while the other half were exposed to dark ones; the situation was reversed in the second phase (Demb et al. 2001, Schwartz et al. 2012, Borghuis et al. 2013). Responses to dark bars (i.e., suppressed excitatory inputs) did not cancel responses to bright ones (i.e., excitatory inputs) in either phase, though, which led to a frequency-doubled excitatory response to a full stimulus cycle (Figure 4).
Figure 4.
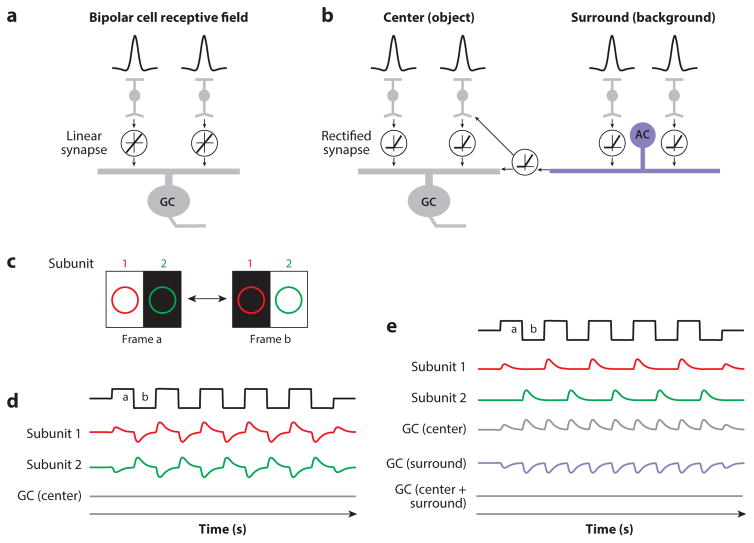
Nonlinear subunit receptive field. (a) In one model, bipolar cells have center–surround spatial receptive fields (top), and their synapses are linear (i.e., equal but opposite changes in release rate occur in response to increments or decrements in light intensity). The subunits are integrated by a ganglion cell (GC). The input–output function illustrated in the circle shows the linear relationship between the presynaptic bipolar cell voltage and glutamate release onto the ganglion cell. (b) In a second model, bipolar cell vesicle release is nonlinear because it is rectified (i.e., release can increase but cannot decrease below a low baseline rate). The input–output function illustrated in the circle shows the nonlinear relationship between presynaptic voltage and glutamate release. The circuit is expanded to have an amacrine cell (AC)-mediated surround inhibition that is driven by bipolar cell subunits. Release from the AC is also rectified; thus, inhibition to the bipolar cell terminals and to the GC is rectified. (c) Alternating frames of a grating stimulus viewed by each of two bipolar cell subunits. Each subunit is excited by the bright bar (subunit 1 for frame a, subunit 2 for frame b). (d ) For the linear model in panel a, responses from the bipolar subunits are canceled when summed at the level of the ganglion cell. (e) For the nonlinear model in panel b, stimulation of only the center of a receptive field generates a frequency-doubled response in the ganglion cell when the responses of rectified, nonlinear subunits are summed. When stimulation is extended to the surround, however, AC inhibition cancels the center excitation.
Nonlinear responses are apparent in the excitatory synaptic input to ganglion cells and can be explained by the bipolar cell inputs (Demb et al. 2001, Schwartz et al. 2012, Borghuis et al. 2013). The nonlinearity of glutamate release is most striking in laminae near the middle of the IPL, adjacent to the boundary between ON and OFF layers (Roska & Werblin 2001, Baden et al. 2013, Borghuis et al. 2013, Ichinose et al. 2014). Because each bipolar cell subunit is small, it is stimulated by high spatial frequencies; the independent activity of each subunit endows the postsynaptic ganglion cell with sensitivity to fine spatial patterns (Demb et al. 2001, Baccus et al. 2008, Schwartz et al. 2012). For some bipolar cells, including the ON bipolar cells presynaptic to mouse ON α cells, the degree of nonlinearity increases with background light intensity (Grimes et al. 2014b). This occurs because the presynaptic bipolar cell hyperpolarizes in bright light, and consequently, transmission from its terminals becomes more rectified (i.e., it does not encode decrements in light intensity with a reduction in release rate because presynaptic Ca channels are closed by the hyperpolarization). As a consequence, the ON α cell converts from being a linear to a nonlinear integrator as background intensity increases.
LATERAL INHIBITION MODIFIES SIGNALING BY VERTICAL EXCITATORY PATHWAYS
A ganglion cell receptive field was classically described with a center-surround organization (Kuffler 1953). In this scenario, the center is mediated by the bipolar cell input described above. The surround is mediated by inhibitory interneurons comprising two groups—horizontal and amacrine cells—and it is exerted both presynaptically and postsynaptically in two distinct parts of the retina. In the outer plexiform layer, the processes of horizontal cells are postsynaptic to photoreceptor terminals, and photoreceptor input to horizontal cells drives surround inhibition of photoreceptors. In the inner plexiform layer, amacrine cells receive excitatory synaptic input from bipolar cells and make inhibitory synapses onto bipolar cell axon terminals and onto the dendrites of other amacrine and ganglion cells.
Horizontal Cells Inhibit Transmission at Photoreceptor–Bipolar Cell Synapses
Horizontal cells are designed to generate classic, linear surround inhibition in the first synaptic layer of the retina (Thoreson & Mangel 2012). Because of their broad and relatively linear integration, horizontal cells cannot encode high spatial frequencies (Zaghloul et al. 2007, Baccus et al. 2008). There are typically one or two classes of horizontal cell: The so-called A-type (or HI in primates) has an axon, and the B-type (HII in primates) does not. Both are GABAergic neurons (Guo et al. 2010, Puller et al. 2014). The dendrites of both A- and B-type horizontal cells are found postsynaptic to cones; the axon terminals of B-type cells are postsynaptic to rods. The mouse retina has only B-type cells. Through a feedback mechanism that remains uncertain—indeed, it has been the subject of significant controversy and debate—depolarization of horizontal cell dendrites suppresses glutamate release from photoreceptors. Horizontal cells are coupled electrically, thereby increasing the area over which they can exert this surround inhibition (Thoreson & Mangel 2012, Weiler et al. 2000). Additionally, GABA released from horizontal cells could hyperpolarize bipolar cells directly, thereby potentiating surround inhibition (Herrmann et al. 2011, Puller et al. 2014).
For some time, the rod- and cone-contacting ends of the A-type horizontal cells were thought to act independently, with each performing feedback inhibition separately. A recent study, however, suggests that a bright, cone-stimulating stimulus over a wide area can depolarize rods in the central region of the stimulus, which causes rod bipolar cells postsynaptic to these rods to hyperpolarize and behave as OFF cells (Szikra et al. 2014). The most parsimonious explanation for this effect is communication between cone-contacting dendrites and rod-contacting axon terminals via the axon. Indeed, the strong surround effect depends on functional cones and horizontal cells. When the cones were disrupted by genetic ablation of their G proteins, or when horizontal cells were shunted optogenetically, the surround effect was abolished. The utility of such inhibition in natural vision, however, is not obvious: The unconventional OFF response of rod bipolar cells apparently operates alongside the conventional ON or OFF responses of the cone bipolar cells. Then, the integration of the unconventional OFF response and a conventional ON response could generate a mixed ON–OFF response in an otherwise conventional ON ganglion cell.
What is known about the mechanism(s) underlying feedback inhibition that originates from horizontal cells? Because the cells are GABAergic, the likely mechanism initially seemed to be conventional synaptic inhibition; however, receptive field surrounds persist when GABA receptors are blocked pharmacologically, which points to the existence of another mode (or modes) of inhibition (Thoreson & Mangel 2012). Two explanations—which are not mutually exclusive—of horizontal cell feedback inhibition have been advanced. The first posits that depolarization of horizontal cells somehow acidifies the synaptic cleft, thereby inhibiting presynaptic Ca channels on photoreceptor terminals. Support for this hypothesis comes from studies of the mammalian retina in which HEPES, a strong proton buffer, was used to disrupt surround inhibition generated in the outer retina and from a recent study in which a genetically encoded pH indicator was expressed in zebrafish cones (Davenport et al. 2008, Wang et al. 2014). The second hypothesis is that hemichannels (i.e., pores formed by connexins—gap junction subunits—expressed only in the horizontal cell membrane) encourage current flow through the synaptic cleft in such a way as to hyperpolarize photoreceptors when horizontal cells depolarize (Thoreson & Mangel 2012, Vroman et al. 2014).
Amacrine Cells Inhibit Other Interneurons and Ganglion Cells
Amacrine cells are classified broadly as either narrow or wide field on the basis of the diameters of their dendritic trees. Narrow-field cells are commonly glycinergic and wide-field cells are commonly GABAergic (Zhang & McCall 2012). In the absence of strong electrical coupling between neighbors, narrow-field cells signal only locally, but many wide-field cells, which extend hundreds of micrometers or even millimeters across the retina, regularly mediate very long-range synaptic interactions and shape the behaviors of inhibitory surrounds significantly (Zaghloul et al. 2007, Baccus et al. 2008). Owing to their size, these wide-field cells generally rely on action potentials to propagate signals across their morphologies, which include axons (Masland 2012, Zhu et al. 2014). Narrow-field amacrine cells seem to fine-tune local glutamate input, including a role in crossover inhibition noted above (Figure 3). The wide-field amacrine cells seem to play a role for surround generation complementary to the horizontal cells. One key difference between horizontal and wide-field amacrine cells is that the amacrine cells are driven by bipolar cells and can therefore exhibit the same sensitivity to high–spatial frequency stimuli as Y ganglion cells (Zaghloul et al. 2007, Baccus et al. 2008) (Figure 4).
Wide-Field Amacrine Circuits Create Strong Nonlinear Surround Cells
The functional components described above explain how ganglion cells could commonly have a nonlinear center-surround receptive field. The center is driven by direct bipolar cell input (excitatory subunits), and the surround is driven by wide-field amacrine cells, which are driven by the same bipolar cells (inhibitory subunits, from the perspective of the ganglion cell; Figure 4b). This receptive field structure emphasizes contrast differences between center and surround regions, with sensitivity to high spatial frequencies at the scale of the bipolar cell subunit receptive fields (50–100 μm wide).
When the amacrine cell input is strong, the inhibition from the surround can null the excitation from the center completely in the ganglion cell. We call ganglion cells that exhibit such responses strong nonlinear surround (SNS) cells. SNS cells should have strong preferences for localized contrast stimuli, which drive the center but not the surround. For example, if a high-contrast stimulus (e.g., a high–spatial frequency grating or a moving spot) is presented over the center alone (with no contrast in the surround), the SNS cells will respond; the same cells will be silenced completely if the stimulus extends into the surround (Figure 4e). This behavior describes the local edge detector (LED) type of ganglion cell described in early in vivo recordings of the rabbit retina (Masland 2012). More recently, the receptive fields of these cells have been explored using whole-cell recordings in both mouse and rabbit retinas, and this work has demonstrated strong surround inhibition of LED cells in either direct (via inhibitory synapses onto LED cells) or indirect (via inhibitory synapses onto presynaptic bipolar cell terminals) inhibitory circuits, depending on species (Russell and Werblin 2010, Zhang et al. 2012, Venkataramani et al. 2014).
This SNS receptive field structure also has been described for so-called object motion sensitive (OMS) cells (Olveczky et al. 2003, Baccus et al. 2008). In a creative set of experiments, a high-contrast, high–spatial frequency grating was jittered over the receptive field center to mimic the effect of small-amplitude eye movements (e.g., microsaccades and eye drift) that occur during voluntary fixation. When a peripheral grating jittered with the same time course as the central grating, the response to the central grating was canceled (Figure 4e). When the central and peripheral stimuli jittered with separate temporal sequences—mimicking genuine object motion that is distinct from eye motion—the OMS cells responded because the excitation and inhibition did not overlap in time. The circuit that explains this effect is the SNS receptive field described above.
There is no feature of the OMS (SNS) circuit that facilitates the detection of motion. Rather, the circuit prevents the response to simultaneous motion across a full field, as occurs during small eye movements when a stationary object on a background is viewed. But this suppressive action occurs only under specific stimulus conditions: when the central object and surrounding background are composed of features (i.e., high contrast, high spatial frequency) that similarly activate the subunits. For a high-contrast object on a low-contrast background, for example, the surround effect would be weak, and both eye motion and object motion would evoke similar responses, negating OMS function. Thus, the concept of OMS deserves further exploration. More generally, to link the function of a ganglion cell with a specific perception or behavior, it will be necessary to inactivate the presumed synaptic mechanism and to determine whether the perception or behavior in question is selectively altered.
The Starburst Amacrine Cell Creates Direction Selectivity
The starburst amacrine cell shows several specializations that enable a direction-selective computation in the retina. Starburst cells can be linked directly to direction selectivity because the DS ganglion cell response is abolished when starburst cells are ablated or temporarily inactivated (Vaney et al. 2012, Vlasits et al. 2014). The starburst amacrine receives excitatory input across its dendritic arbor but makes output synapses only at its dendritic tips (Vaney et al. 2012). Its primary dendrites are thin, and each presynaptic active zone is isolated electrotonically and acts independently. Furthermore, each active zone is, on average, direction selective in that it responds most strongly to motion in the direction from the soma to the tip (Euler et al. 2002, Yonehara et al. 2013). DS release arises from intrinsic properties of the cells and from inhibitory interactions between cells (Vaney et al. 2012).
A recent connectomic and modeling study of the OFF starburst cell suggested a novel excitatory mechanism for DS: The OFF starburst cell apparently receives sustained bipolar cell inputs near the soma and transient bipolar cell inputs near the dendritic tips (Kim et al. 2014). When motion moves outward from the center toward the tip, the transient and sustained bipolar inputs would maximally depolarize the release sites at the tips. A physiological confirmation of this model awaits further study.
The starburst cells generate direction selectivity in DS ganglion cells through specific synaptic connections. There are multiple mosaics of DS ganglion cells, each with either ON–OFF or ON responses that prefer one of four motion directions (ON–OFF DS cells: up, down, backward, forward) or one of three directions (ON DS cells) (Vaney et al. 2012). Selective wiring with starburst cell dendrites determines the direction preference of the DS ganglion cell. For example, a DS cell that prefers upward motion receives inhibitory inputs that prefer downward motion (i.e., downward-pointing starburst amacrine cell dendrites) (Briggman et al. 2011).
Originally, measurements of excitatory currents were used to support the proposition that DS ganglion cells received DS excitation with a preferred direction opposite that of DS inhibition (Vaney et al. 2012). There are, however, concerns that measurements of DS excitation could be compromised by poor voltage control of large, electrotonically complex DS ganglion cells (Poleg-Polsky & Diamond 2011), and direct measurements of glutamate release with iGluSnFR suggest that excitatory transmission onto DS ganglion cells is not direction selective (Yonehara et al. 2013, Park et al. 2014). Thus, the primary synaptic mechanism for generating DS apparently resides within the inhibitory circuitry. Intrinsic properties of the DS ganglion cell—most notably the generation of dendritic action potentials in response to local excitation—amplify DS (Oesch et al. 2005, Sivyer & Williams 2013). Additionally, electrical coupling within one class of DS ganglion cell amplifies the DS within the network and generates responses in separated neurons with constant lag times (Trenholm et al. 2013). Size tuning is achieved separately from direction tuning via feedback from wide-field amacrine cells onto the presynaptic bipolar terminals of the DS cell (Hoggarth et al. 2015).
Interestingly, DS appears modifiable: The DS preference of a mouse ganglion cell assessed in vitro was shown to reverse following prolonged visual stimulation (Rivlin-Etzion et al. 2012). This reversal in DS preference was accompanied by a reversal of starburst amacrine cell polarity; e.g., an ON starburst cell responded like an OFF cell (Vlasits et al. 2014). This effect was seen only in the superior retina, which in the mouse contains cones with primarily M opsin expression (Figure 2a), and the effect might be explained by a suppression of photoreceptor function by the bright stimulus so that the responses of starburst cells were dominated by inputs from their surrounds. It is not known whether the reversed responses of DS and starburst cells occur in vivo, where the retina can regenerate photopigment and is therefore less prone to bleaching.
The A17 Amacrine Cell Possesses Multiple, Independent Feedback Circuits
Not all amacrine cell structures match their functions. For example, the A17 amacrine cell appears to be a wide-field cell that receives input from rod bipolar cells on varicosities spaced at ~1-μm increments on very thin dendrites (Nelson & Kolb 1985, Grimes et al. 2014a). At the same sites, the A17 makes reciprocal GABAergic synapses onto the same rod bipolar cells. Similar to active zones in starburst cells, the varicosities on A17s are electrotonically isolated, which makes feedback inhibition a localized phenomenon (Grimes et al. 2010). Perhaps to ensure this isolation and localization, release from A17 varicosities is triggered by Ca2+ influx through postsynaptic Ca2+-permeable AMPARs rather than voltage-gated Ca channels (Chavez et al. 2006). Conventional voltage-gated Ca channels appear to exist in A17 dendrites, but colocalized Ca2+-activated K channels limit their influence on membrane potential (Grimes et al. 2009).
Finally, other synaptic motifs serve local integration of bipolar and amacrine inputs. One is feedforward inhibition, which acts in parallel with excitation to suppress excessive depolarization in the face of high release rates (Figure 3b); this improves synaptic signal-to-noise ratios (Cafaro & Rieke 2010, Demb & Singer 2012). A second is crossover (usually ON to OFF pathway) inhibition, which converges with excitatory circuits at ganglion cell dendrites (Figure 3b). For example, an OFF α cell integrates OFF excitatory input from presynaptic OFF bipolar cells and ON inhibitory input from presynaptic ON amacrine cells, including the AII amacrine cell (Figure 1b) (Demb & Singer 2012, Ke et al. 2014). The two pathways combine to generate depolarization at light OFF and hyperpolarization at light ON. The rate of spontaneous activity at OFF bipolar cell synapses is low, which causes a rectification to light increments (i.e., release rate cannot be negative) (Borghuis et al. 2013). The addition of active ON inhibitory inputs, however, serves to make the net synaptic input to the cell less rectified: The magnitudes of ON hyperpolarization and OFF depolarization become more evenly matched (Werblin 2010). In primate parasol cells, crossover inhibition shapes the temporal response to excitatory inputs during stimulation with contrast patterns (Cafaro & Rieke 2013). In salamander, a circuit for disinhibition was discovered that operates within a single (OFF) pathway (Manu & Baccus 2011).
Inhibitory Circuit Mechanisms Mediate Color Opponency
L versus M opponency in the primate fovea apparently reflects the fact that the excitatory centers of foveal midget ganglion cells are driven by single cones (L or M), whereas the inhibitory surrounds of these cells are driven by multiple cones, many of the class opposite the one that drives the center. The result is a center/surround receptive field with net L–M (or M–L) opponency (Lee et al. 2010). In the periphery, however, midget cell centers are driven by multiple cones, and there has been no evidence of selective wiring that could explain how opponency is preserved (Crook et al. 2011). Using a multielectrode array recording of peripheral retina and small stimuli that localized each cone location and spectral type, it was shown that there is a weak but significant bias across midget cells (Field et al. 2010). The bias caused a higher than chance weighting of L (or M) inputs to the centers in a subset of midget ganglion cells combined with a mixed L/M surround. The center–surround combination generates color opponency across the midget cell mosaic in a subset of cells.
Two mechanisms might allow a small bistratified cell in primate retina to be S versus L/M opponent: S cones themselves are color-opponent because they receive horizontal cell feedback mediated by L/M cones (Packer et al. 2010, Dacey et al. 2014, Puller et al. 2014), and the bistratified cell integrates inputs from L/M-cone-driven OFF bipolar cells and the S-cone-driven ON bipolar cells (Crook et al. 2009).
A novel OFF S versus L/M mechanism was recently described in the ground squirrel. Here, the ganglion cell receptive field is shaped by feedforward inhibition (see above) that arises from amacrine cells alone—a sign-inverting circuit (Figure 3b). An ON-type S-cone bipolar cell with an M-cone surround makes synapses with a glycinergic amacrine cell that contacts ganglion cells: The amacrine cell is excited by blue light, but excitation of the amacrine cell inhibits the postsynaptic ganglion cells. Thus, the ON response is inverted to an OFF response and its color-opponent nature is preserved (Chen & Li 2012, Sher & DeVries 2012). The above mechanisms for conveying S-cone signals to ganglion cells are apparently complemented by OFF-type S-cone bipolar cells in some species (Klug et al. 2003, Yin et al. 2009, Mills et al. 2014).
Mechanisms for Lateral and Crossover Excitation
It is worth mentioning that lateral interactions can also be excitatory. This starts at the first retinal synapse, as excitation can spread between photoreceptors through electrical synapses (Bloomfield & Völgyi 2009) as well as via glutamate diffusion between neighboring synaptic clefts, which can have a depolarizing effect (Szmajda & DeVries 2011). Glutamate also can spill over at the level of bipolar cell axon terminals, but in this case, the effect is hyperpolarizing owing to a glutamate transporter–associated anion current (Veruki et al. 2006). Electrical signals spread laterally in the inner retina, across gap junctions between ganglion and amacrine cells (Pan et al. 2010). These signals can coordinate correlated firing between cells receiving coincident chemical synaptic input. The correlated spikes provide information about stimulus qualities that is independent of the firing-rate modulations driven by conventional receptive field mechanisms (Trenholm et al. 2014).
Additionally, the VGluT3+ amacrine cell plays an unconventional role by directly exciting some ganglion cell types via glutamate release (Lee et al. 2014, Kim et al. 2015). These cells have ON-OFF receptive fields with strong surround inhibition. Thus, to small stimuli, they will convey an ON-OFF excitation to postsynaptic ganglion cells. Optogenetic stimulation showed that postsynaptic targets include ON–OFF DS cells, ON DS cells, and LED cells. The VGluT3+ cell makes synapses in both ON and OFF layers; therefore, it is capable of crossover excitation by delivering an OFF excitatory input to an ON DS cell.
ADAPTATION
Vertebrate vision encompasses billion-fold changes in stimulus intensity, from dim starlight to bright sunlight (e.g., Shapley & Enroth-Cugell 1984). A neuron typically has an operating range that covers only ~1–2 log units, so the encoding of widely varying input requires adaptation. Adaptation within the retina arises in photoreceptors (i.e., receptor adaptation) and in the inner retina (i.e., postreceptor, or neural, adaptation). Receptor adaptation is the slower process; it is driven largely by changes in ambient light intensity. The mechanisms of receptor adaptation have been studied and reviewed extensively [e.g., cones (Schneeweiss & Schnapf 1999) and rods (Fain & Matthews 1990)]; here, we focus on neural adaptation that occurs in the inner retina (i.e., in bipolar cells and in their postsynaptic circuits).
Neural adaptation is driven by multiple components of the visual scene, including the mean light level (i.e., background) and the variance of light around the mean (i.e., temporal contrast). Network sensitivity—gain—increases when background and/or temporal contrast are low, to maintain a high signal-to-noise ratio, and decreases when background and/or temporal contrast are high, to avoid response saturation. The speed and flexibility of neural adaptation must be appreciated. Even within a single daytime scene, local intensities can exhibit tremendous—~10,000-fold—variability (e.g., Reinagel & Zador 1999). As the action potential firing frequency of individual ganglion cells can change by only ~100-fold, to encode visual stimuli with high fidelity as eye position varies and the visual scene changes, the retinal circuit adapts rapidly to match the ganglion cell spiking outputs to the dynamic range of the local scene statistics (e.g., Dunn & Rieke 2006, Demb 2008).
Synaptic Depression Generates Visual Adaptation
Over the course of the past 10–15 years, a general principle governing neural adaptation in the retina has emerged. Adaptation to background and to temporal contrast share a common mechanism—a use-dependent decrease in local excitability. This decrease arises from the properties of retinal synapses, particularly the excitatory synapses of bipolar cells, and is augmented by the intrinsic membrane properties of retinal neurons. The implementation of neural adaptation in bipolar cell axon terminals allows the retinal network to process a visual scene effectively by adapting locally, at the scale of a bipolar cell receptive field.
Neural adaptation to background luminance reflects use-dependent depression of synaptic transmission from bipolar cells. For both mouse rod and primate cone pathways, backgrounds that are insufficient to cause receptor adaptation reduce light flash–evoked synaptic currents recorded in ganglion cells. In the case of the rod pathways, neural adaptation occurs at backgrounds as low as 1–2 rhodopsin isomerizations [R*]/(rod s) (Dunn et al. 2006). Synaptic currents recorded in primate ganglion cells and evoked by cone stimulation show adaptation to backgrounds that produce as few as 100 R*/(cone · s) (Dunn et al. 2007). Such low backgrounds do not affect the voltage responses of bipolar cells to the same light flashes, indicating that adaptation reflects a change in the strength of the bipolar cell synapses. These changes are reflected in synaptic currents recorded from AII amacrine cells postsynaptic to rod bipolar cells and from ganglion cells postsynaptic to cone bipolar cells (Dunn et al. 2006, 2007; Dunn & Rieke 2008).
How does adaptation to background arise at bipolar cell synapses? In an elegant experiment, Dunn & Rieke (2008) recorded from a synaptically coupled pair comprising a presynaptic rod bipolar cell and a postsynaptic AII amacrine cell, and they demonstrated that a background of 1 R*/(rod ·s) depressed transmission at the synapse evoked by a brief presynaptic stimulus that mimicked a light flash. This result suggests that depolarization of the rod bipolar cell, by the background light, increased tonic vesicle release at its synapses, thereby depleting the readily releasable vesicle pool and reducing the number of vesicles available to undergo exocytosis following a brief presynaptic stimulus (Figure 6). This finding is consistent with previous studies of the rod bipolar synapse that demonstrated the existence of a small, readily releasable vesicle pool with high release probability and a propensity toward profound short-term synaptic depression (Singer & Diamond 2006). More recent studies characterize the relationship between background light intensity, presynaptic membrane potential, and tonic synaptic transmission, and they confirm that sustained depolarization of the rod bipolar cell by background light depletes the readily releasable vesicle pool and diminishes transmission evoked by brief stimuli ( Jarsky et al. 2011, Oesch & Diamond 2011). The depolarization-dependent suppression of synaptic transmission is augmented by voltage-dependent inactivation of presynaptic Ca channels ( Jarsky et al. 2011, Grimes et al. 2014b, Ke et al. 2014).
Figure 6.
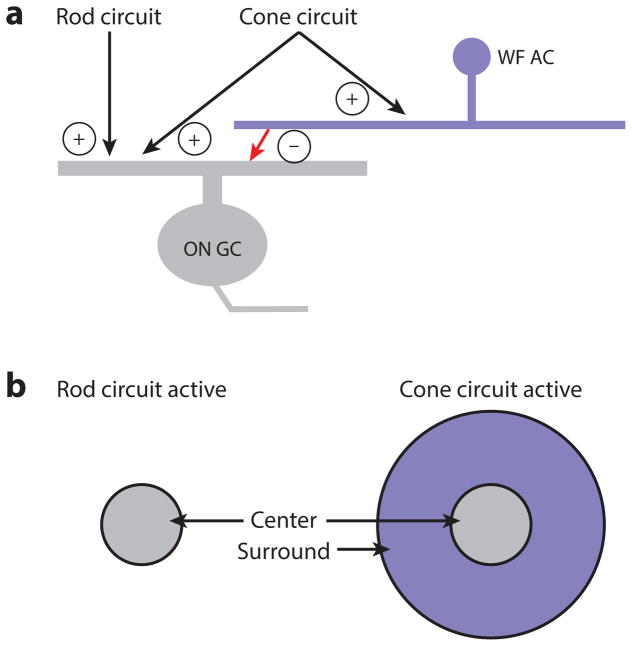
Example of circuit switching. (a) The excitatory input to an ON ganglion cell (GC) is driven by both rod and cone circuits. The rod circuits actually signal via the cone bipolar cell terminal (see Figure 1). The inhibition from the surround is mediated by a wide-field amacrine cell (WF AC) driven exclusively by cone circuits. (b) When the rod circuit is active, the ON GC has a receptive field with an excitatory center component only. When the cone circuit is active, the inhibitory surround component switches on.
Neural adaptation to temporal contrast is studied by examining neural responses to a time-varying (filtered white noise) stimulus in which the intensity is varied around a mean; a linear–nonlinear analysis is used to quantify changes in response gain (i.e., adaptation) (Demb 2008). Temporal contrast adaptation must be implemented in the inner retina (i.e., in bipolar and/or amacrine cells) because it is not apparent in photoreceptors or in one of their postsynaptic targets, horizontal cells (Baccus & Meister 2002, Demb 2008). Indeed, studies of the salamander retina have suggested that both excitatory and inhibitory postreceptoral mechanisms can contribute to contrast adaptation; however, specific circuit components that can explain adaptation across all of these studies do not exist (Demb 2008, Bölinger & Gollisch 2012, Garvert & Gollisch 2013).
Studies of ganglion cells in primate, guinea pig, and salamander retinas demonstrate that temporal contrast adaptation involves synaptic and postsynaptic components (Demb 2008, Ozuysal & Baccus 2012, Garvert & Gollisch 2013). The synaptic component arises from a use-dependent reduction in the strength of—or even suppression of—excitatory synaptic inputs from bipolar cells to ganglion cells independent of presynaptic inhibition (Demb et al. 2001, Demb 2008); the postsynaptic component reflects activity-dependent changes in Na and K conductances that govern action potential generation by ganglion cells (Demb 2008, Weick & Demb 2011).
The reduction in the strength of transmission from bipolar cells is accounted for by the same mechanism that is responsible for adaptation to background: primarily, a reduction in the readily releasable vesicle pool ( Jarsky et al. 2011). As the time-varying distribution of bipolar cell membrane potential changes with the visual scene, bipolar cell synapses experience activity-dependent changes, and adaptations to background and contrast arise in postsynaptic neurons; in very general terms, depolarized rod bipolar cells cannot encode transient stimuli ( Jarsky et al. 2011, Oesch & Diamond 2011, Ke et al. 2014). This general notion is supported by a systems-level analysis of temporal contrast adaptation in which changes in the gain of ganglion cell responses were well described by a resource allocation model, the parameters of which were similar to the known properties of cycling vesicle pools in bipolar cell terminals (Ozuysal & Baccus 2012). Thus, to a first approximation, temporal contrast adaptation is simply adaptation to the mean intensity of a rectified stimulus.
Importantly, this common mechanism underlying adaptation to background and to temporal contrast reflects the fact that recovery of bipolar cell synapses from short-term depression is far slower than entry into the depressed state (depletion of the readily releasable pool in <20 ms versus recovery in ~1 s) (Mennerick & Matthews 1996, Singer & Diamond 2006, Ke et al. 2014). Thus, the vesicle recycling rate limits the number of available vesicles during periods of depolarization. As the presynaptic membrane spends more time at depolarized potentials (because of either a depolarized mean potential or frequent large, transient depolarizations), the readily releasable pool shrinks and synaptic gain is reduced ( Jackman et al. 2009, Odermatt et al. 2012).
It is important to note that temporal contrast adaptation generally has been studied using spatially uniform (i.e., full-field) stimuli. However, if adaptation depended on a mechanism in bipolar cells, it would be expected to occur within the subunits of a larger receptive field (Figure 4). Interestingly, the majority of ganglion cells in the salamander retina appear to possess nonlinear receptive fields (Bölinger & Gollisch 2012). And, in the salamander retina, contrast adaptation in a subset of these ganglion cells depends strongly on local nonlinearities (Garvert & Gollisch 2013).
Several recent studies have extended investigation of contrast adaptation to examine local circuit processing within ganglion cell receptive fields. These studies have identified interactions between excitatory bipolar cell and inhibitory amacrine cell synapses that provide an additional layer of processing to expand the behavioral repertoire of ganglion cells.
Bölinger & Gollisch (2012) found that some salamander ganglion cells responded more strongly than others to spatially homogeneous stimuli. The outputs of these neurons appeared to depend strongly on the activity of inhibitory amacrine cells, which suppress ganglion cell spiking in the presence of strong local stimulation. Local inhibition also is responsible for a phenomenon termed sensitization, in which a ganglion cell responds more strongly to a low-contrast stimulus following exposure to a high-contrast one. Subsets of ganglion cells in both salamander and mouse retinas exhibit this phenomenon. It has been proposed that sensitization results from inhibitory amacrine cells adapting to contrast more strongly than do excitatory bipolar cells (Kastner & Baccus 2011, Nikolaev et al. 2013). Sensitization can arise within subunits of the receptive field of a ganglion cell, indicating that local, use-dependent regulation of the strength of both excitatory and inhibitory synapses is a critical determinant of ganglion cell output. Presumably, such locally tuned synaptic transmission permits ganglion cells to encode visual stimuli more precisely (Kastner & Baccus 2013). The extent to which such retinal computations are propagated to higher stages of visual processing raises interesting questions (e.g., do neurons in primary visual cortex exhibit behaviors that can be attributed to retinal sensitization?) that can be addressed in the future (Solomon & Kohn 2014).
Intensity-Dependent Circuit Switching
Ganglion cells are commonly described as having static receptive fields, but some of their basic receptive field properties can change—beyond adaptive gain controls—with the lighting conditions. For example, ON and OFF α ganglion cells in the mouse lose their inhibitory surrounds in dim light because some inhibitory amacrine cells are driven poorly by rod circuits. The inhibitory circuit underlying the surround seems to switch on at a threshold for cone vision. There is circumstantial, not direct anatomical or electrophysiological, evidence that these amacrine cells are electrically coupled to cone bipolar cells such that weak cone bipolar cell responses in dim light fail to activate the amacrine cells (Farrow et al. 2013) (Figure 6). It is difficult to understand how any circuit-level interactions in the inner retina could be activated exclusively at the cone threshold, however, because rods and cones share so much circuitry. Indeed, rods signal to ganglion cells exclusively through cones and cone bipolar cells (Figure 1b), and transmission through cone bipolar cell terminals is modulated strongly over the wide dynamic range of rod vision (Grimes et al. 2014b, Ke et al. 2014). How would a postsynaptic amacrine cell distinguish between rod- and cone-based drive of the same presynaptic cone bipolar terminal? Furthermore, in the mouse retina, a cone-dependent surround mechanism would depend strongly on the wavelength of light relative to the gradient of cone opsin expression and therefore would vary across retinal location (Figure 2). Thus, the mechanism and wavelength dependence of circuit-switching surrounds requires further study.
The corollary to the observation of a surround driven only by cone stimulation is the notion that rod pathways turn off in bright light. The specialized rod bipolar/AII amacrine cell pathway might be expected to cease to function during cone vision because electrical coupling within it could impair spatial acuity. For example, it had been proposed that the strength of electrical coupling in the AII network would be turned down in bright light (e.g., Bloomfield & Völgyi 2009). Rod bipolar cells, however, can continue to respond in background light so long as the visual input generates alternating depolarizing and hyperpolarizing responses (i.e., Michelson contrast); under these conditions, hyperpolarization relieves vesicle depletion enabling a burst of release at the subsequent depolarization (Ke et al. 2014). The AII amacrine cell also contributes to vision in bright light, in which it can be driven through gap junctions with ON cone bipolar cells and directly inhibit OFF ganglion cells (Demb & Singer 2012). Thus, some interneurons can repurpose their functions across lighting conditions.
CONCLUSION
Arguably, we understand the retina better than other parts of the mammalian central nervous system. To solve the retina, however, the field needs still to complete the anatomical identification and functional characterization of cell types; this is especially pressing for the amacrine and the ganglion cells. Genetics-based tools will provide exciting experimental opportunities in the future. One would like genetic access to individual cell types so that their function can be altered selectively—either optogenetically or via designer drug-receptor pathways—while the activities of other neurons in the circuit are assessed electrophysiologically (e.g., Beier et al. 2013, Duan et al. 2014, Vlasits et al. 2014, Zhu et al. 2014). Retinal neurobiology will also benefit from recent advances in large-scale anatomical reconstruction (Helmstaedter et al. 2013). In particular, connectomics has the potential to elucidate circuit function (e.g., Briggman et al. 2011) and to provide testable hypotheses about newly discovered circuits (Marc et al. 2013).
Signaling in bipolar cells is fairly well understood. The same cannot be said for signaling by the inhibitory amacrine cells that modulate bipolar cell output and ganglion cell excitability. Apart from just a few identified cell types with well-characterized functions (e.g., starburst amacrine cells, AII amacrine cells, and A17 amacrine cells), the intrinsic properties, synaptic dynamics, and circuit functions of most of the 40–50 amacrine cell types in the mammalian retina are mysterious. For example, three distinct cell types with unique morphology and physiology were identified within the class of vasoactive intestinal polypeptide–expressing amacrine cells (Park et al. 2015). In the future, a primary goal of retinal neurophysiology should be to study lateral inhibitory circuitry so that it is explained to the same extent as the vertical excitatory pathways that we have reviewed here.
Finally, we must consider the fact that although the retina is designed to function over an enormous range of stimulus intensities, generally it is studied over only a small portion of its operating range. Recent studies illustrate, albeit qualitatively, how little we understand about the behaviors of some cell types in response to changing ambient light intensities. Across ~100-fold changes in background light level, some ON or OFF cells seemed to alter their identities and generate ON–OFF responses (Pearson & Kerschensteiner 2015, Tikidji-Hamburyan et al. 2015). These effects were also observed in vivo. The mechanism remains incompletely understood but likely relates to some of the circuit switching mechanisms noted above, including horizontal cell–mediated OFF responses in rods and crossover excitation from the VGluT3+ cell. Understanding how the retinal code changes across lighting conditions remains a challenge for the field.
Figure 5.
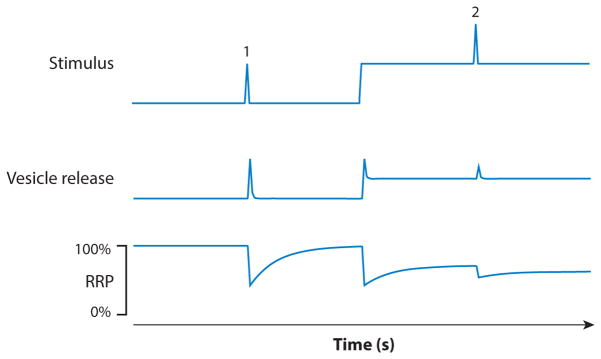
Synaptic mechanism for adaptation. A test pulse is presented in darkness (pulse 1) or added to a background (pulse 2, top trace). The first test pulse evokes a burst of vesicle release. When the background is added, tonic vesicle release increases, and the response to a second test pulse is reduced relative to the first. The readily releasable pool (RRP) of synaptic vesicles is decreased by both the test pulses and the background stimulus. Recovery from depletion of the RRP is relatively slow compared with the decay of release following the test pulse. The result is that a background light depletes the RRP and causes adaptation.
SUMMARY POINTS.
The retina is one of the best understood regions of the central nervous system; it comprises an estimated 100 distinct cell types that form ~20–30 circuits that terminate onto the same number of ganglion cells (output neurons).
Retinal computation depends on a dynamic balance of synaptic excitation and inhibition regulated by use-dependent depression applied differentially to various parallel pathways that feed ganglion cells.
The mouse retina shows regional changes in expression of cone opsins, and the ventral portion of the mouse retina has a strong sensitivity to UV light. The highest densities of the smallest members of different ganglion cell types are located in different regions of the retina, allowing cell type–specific acuity for viewing different parts of the same visual scene.
Parallel pathways of excitatory bipolar cells depend on specific glutamate receptors, voltage-gated channels, presynaptic release properties, and inhibitory circuits to create unique temporal processing channels for signaling either increments or decrements in light intensity.
Nonlinear properties of transmission from bipolar cell terminals generate nonlinear center–surround receptive fields in ganglion cells sensitive to regional contrast variations.
Amacrine cells have specialized intrinsic and circuit properties that contribute to feature selectivity, including direction selectivity and color opponency. Some wide-field amacrine cells perform multiple independent local processing computations within a single dendritic tree.
Adaptation depends on synaptic depression at the output of bipolar cells. This mechanism explains adaptation to both the mean light level and the contrast level.
Some components of retinal circuitry alter their functions in a light-dependent manner. This switching can alter the balance of excitatory and inhibitory input within a circuit or can repurpose a single neuron for a second role at the new light level.
FUTURE ISSUES.
Complete identification of the ~100 cell types in the retina will be useful because it will give the field a common language for describing results across laboratories.
Large-scale electron micrograph reconstruction is helping to understand how the ~100 retinal cell types are wired together into functional circuits. Future advances will include automated algorithms for identifying cell boundaries and synapses within large tissue volumes.
The basic properties of many types of inhibitory interneurons—namely, the amacrine cells—require further study with regard to basic light responses, morphology, and connections to other retinal neurons.
Gaining genetic access to individual cell types within the retina will be important, as such access will allow experimenters to determine the role(s) played by single cell types in circuit function and behavior via activating or inactivating them.
It will be important to determine the homology of cell types between species, especially that between mouse and primate, in terms of gene expression, physiology, and role in circuit function.
The field needs to continue to better understand how retinal circuit function varies with background light level. Recent studies show qualitatively how a cell can change identity across light levels—from encoding light increments to encoding both increments and decrements—but the mechanism is not well understood.
Acknowledgments
The authors are supported by grants from the National Eye Institute: EY014454 to J.B.D., EY017836 to J.H.S., and EY021372 to J.B.D. and J.H.S.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–23. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Asari H, Meister M. Divergence of visual channels in the inner retina. Nat Neurosci. 2012;15:1581–89. doi: 10.1038/nn.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–19. doi: 10.1016/s0896-6273(02)01050-4. [DOI] [PubMed] [Google Scholar]
- Baccus SA, Olveczky BP, Manu M, Meister M. A retinal circuit that computes object motion. J Neurosci. 2008;28:6807–17. doi: 10.1523/JNEUROSCI.4206-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Esposti F, Nikolaev A, Lagnado L. Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision. Curr Biol. 2011;21:1859–69. doi: 10.1016/j.cub.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden T, Schubert T, Chang L, Wei T, Zaichuk M, et al. A tale of two retinal domains: near-optimal sampling of achromatic contrasts in natural scenes through asymmetric photoreceptor distribution. Neuron. 2013;80:1206–17. doi: 10.1016/j.neuron.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Baylor D. How photons start vision. PNAS. 1996;93:560–65. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Borghuis BG, El-Danaf RN, Huberman AD, Demb JB, Cepko CL. Transsynaptic tracing with vesicular stomatitis virus reveals novel retinal circuitry. J Neurosci. 2013;33(1):35–51. doi: 10.1523/JNEUROSCI.0245-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Schwartz GW, Turner MH, Rieke F, Wong RO. Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Curr Biol. 2014;24:310–15. doi: 10.1016/j.cub.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölinger D, Gollisch T. Closed-loop measurements of iso-response stimuli reveal dynamic nonlinear stimulus integration in the retina. Neuron. 2012;73:333–46. doi: 10.1016/j.neuron.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Looger LL, Tomita S, Demb JB. Kainate receptors mediate signaling in both transient and sustained OFF bipolar cell pathways in mouse retina. J Neurosci. 2014;34:6128–39. doi: 10.1523/JNEUROSCI.4941-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Marvin JS, Looger LL, Demb JB. Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina. J Neurosci. 2013;33:10972–85. doi: 10.1523/JNEUROSCI.1241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Ratliff CP, Smith RG, Sterling P, Balasubramanian V. Design of a neuronal array. J Neurosci. 2008;28:3178–89. doi: 10.1523/JNEUROSCI.5259-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–88. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Euler T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J Neurophysiol. 2011;105:2601–9. doi: 10.1152/jn.00722.2010. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–88. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Buldyrev I, Puthussery T, Taylor WR. Synaptic pathways that shape the excitatory drive in an OFF retinal ganglion cell. J Neurophysiol. 2012;107:1795–807. doi: 10.1152/jn.00924.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Rieke F. Noise correlations improve response fidelity and stimulus encoding. Nature. 2010;468:964–67. doi: 10.1038/nature09570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Rieke F. Regulation of spatial selectivity by crossover inhibition. J Neurosci. 2013;33:6310–20. doi: 10.1523/JNEUROSCI.4964-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–8. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Chen S, Li W. A color-coding amacrine cell may provide a blue-Off signal in a mammalian retina. Nat Neurosci. 2012;15:954–56. doi: 10.1038/nn.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. J Physiol. 1971;217:473–96. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Parallel circuits from cones to the on-beta ganglion cell. Eur J Neurosci. 1992;4:506–20. doi: 10.1111/j.1460-9568.1992.tb00901.x. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Graham D, Nelson R. Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina. J Comp Neurol. 2004;477:371–85. doi: 10.1002/cne.20261. [DOI] [PubMed] [Google Scholar]
- Crook JD, Davenport CM, Peterson BB, Packer OS, Detwiler PB, Dacey DM. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. J Neurosci. 2009;29:8372–87. doi: 10.1523/JNEUROSCI.1218-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red–green” color opponency in midget ganglion cells of the primate retina. J Neurosci. 2011;31:1762–72. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook JD, Packer OS, Dacey DM. A synaptic signature for ON- and OFF-center parasol ganglion cells of the primate retina. Vis Neurosci. 2014;31:57–84. doi: 10.1017/S0952523813000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Pan ZH. Two types of cone bipolar cells express voltage-gated Na+ channels in the rat retina. Vis Neurosci. 2008;25:635–45. doi: 10.1017/S0952523808080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Crook JD, Packer OS. Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina. Vis Neurosci. 2014;31:139–51. doi: 10.1017/S0952523813000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008;28:456–64. doi: 10.1523/JNEUROSCI.2735-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Villa P, Vaquero CF, Kaneko A. Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur J Neurosci. 1998;10:317–23. doi: 10.1046/j.1460-9568.1998.00051.x. [DOI] [PubMed] [Google Scholar]
- Demb JB. Functional circuitry of visual adaptation in the retina. J Physiol. 2008;586:4377–84. doi: 10.1113/jphysiol.2008.156638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Intrinsic properties and functional circuitry of the AII amacrine cell. Vis Neurosci. 2012;29:51–60. doi: 10.1017/S0952523811000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J Neurosci. 2001;21:7447–54. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–56. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–48. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Dhande OS, Stafford BK, Lim A, Huberman AD. Retinal ganglion cell and subcortical contributions to visual feature selectivity. Annu Rev Vis Sci. 2015;1:291–328. doi: 10.1146/annurev-vision-082114-035502. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–81. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, de la Huerta I, Sanes JR. Type II cadherins guide assembly of a direction-selective retinal circuit. Cell. 2014;158:793–807. doi: 10.1016/j.cell.2014.06.047. [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–44. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci. 2006;26:3959–70. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Lankheet MJ, Rieke F. Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature. 2007;449:603–6. doi: 10.1038/nature06150. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. The impact of photoreceptor noise on retinal gain controls. Curr Opin Neurobiol. 2006;16:363–70. doi: 10.1016/j.conb.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. Single-photon absorptions evoke synaptic depression in the retina to extend the operational range of rod vision. Neuron. 2008;57:894–904. doi: 10.1016/j.neuron.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Wong RO. Wiring patterns in the mouse retina: collecting evidence across the connectome, physiology and light microscopy. J Physiol. 2014;592:4809–23. doi: 10.1113/jphysiol.2014.277228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci. 2011;28:95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966;187:517–52. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–52. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci. 2014;15:507–19. doi: 10.1038/nrn3783. [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990;13:378–84. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, et al. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron. 2013;78:325–38. doi: 10.1016/j.neuron.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Field GD, Gauthier JL, Sher A, Greschner M, Machado TA, et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010;467:673–77. doi: 10.1038/nature09424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed MA, Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci. 1988;8:2303–20. doi: 10.1523/JNEUROSCI.08-07-02303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvert MM, Gollisch T. Local and global contrast adaptation in retinal ganglion cells. Neuron. 2013;77:915–28. doi: 10.1016/j.neuron.2012.12.030. [DOI] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron. 2010;65:150–64. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Hoon M, Briggman KL, Wong RO, Rieke F. Cross-synaptic synchrony and transmission of signal and noise across the mouse retina. eLife. 2014a;3:e03892. doi: 10.7554/eLife.03892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chavez AE, Diamond JS. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci. 2009;12:585–92. doi: 10.1038/nn.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Schwartz GW, Rieke F. The synaptic and circuit mechanisms underlying a change in spatial encoding in the retina. Neuron. 2014b;82:460–73. doi: 10.1016/j.neuron.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–85. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol. 2010;518:1647–69. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, et al. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–45. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–74. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, et al. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–10. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S, Shapley RM. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976;262:237–64. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggarth A, McLaughlin AJ, Ronellenfitch K, Trenholm S, Vasandani R, et al. Specific wiring of distinct amacrine cells in the directionally selective retinal circuit permits independent coding of direction and size. Neuron. 2015;86(1):276–91. doi: 10.1016/j.neuron.2015.02.035. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–83. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Bi A, Pan ZH. Differential expression of three T-type calcium channels in retinal bipolar cells in rats. Vis Neurosci. 2009;26:177–87. doi: 10.1017/S0952523809090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–38. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Fyk-Kolodziej B, Cohn J. Roles of ON cone bipolar cell subtypes in temporal coding in the mouse retina. J Neurosci. 2014;34:8761–71. doi: 10.1523/JNEUROSCI.3965-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Shields CR, Lukasiewicz PD. Sodium channels in transient retinal bipolar cells enhance visual responses in ganglion cells. J Neurosci. 2005;25:1856–65. doi: 10.1523/JNEUROSCI.5208-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH. Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci. 2009;12:303–10. doi: 10.1038/nn.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Neitz J, Deegan JF., 2nd Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991;353:655–56. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Williams GA. Contributions of the mouse UV photopigment to the ERG and to vision. Doc Ophthalmol. 2007;115:137–44. doi: 10.1007/s10633-007-9055-z. [DOI] [PubMed] [Google Scholar]
- Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, et al. A synaptic mechanism for retinal adaptation to luminance and contrast. J Neurosci. 2011;31:11003–15. doi: 10.1523/JNEUROSCI.2631-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS, Carroll RC, Nawy S. Light-induced plasticity of synaptic AMPA receptor composition in retinal ganglion cells. Neuron. 2012;75:467–78. doi: 10.1016/j.neuron.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Benardete E. The dynamics of primate retinal ganglion cells. Prog Brain Res. 2001;134:17–34. doi: 10.1016/s0079-6123(01)34003-7. [DOI] [PubMed] [Google Scholar]
- Kastner DB, Baccus SA. Coordinated dynamic encoding in the retina using opposing forms of plasticity. Nat Neurosci. 2011;14:1317–22. doi: 10.1038/nn.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner DB, Baccus SA. Spatial segregation of adaptation and predictive sensitization in retinal ganglion cells. Neuron. 2013;79:541–54. doi: 10.1016/j.neuron.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke JB, Wang YV, Borghuis BG, Cembrowski MS, Riecke H, et al. Adaptation to background light enables contrast coding at rod bipolar cell synapses. Neuron. 2014;81:388–401. doi: 10.1016/j.neuron.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–82. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, et al. Space–time wiring specificity supports direction selectivity in the retina. Nature. 2014;509:331–36. doi: 10.1038/nature13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Soto F, Kerschensteiner D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. eLife. 2015;4:e08025. doi: 10.7554/eLife.08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug K, Herr S, Ngo IT, Sterling P, Schein S. Macaque retina contains an S-cone OFF midget pathway. J Neurosci. 2003;23:9881–87. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Marshak D. The midget pathways of the primate retina. Doc Ophthalmol. 2003;106:67–81. doi: 10.1023/a:1022469002511. [DOI] [PubMed] [Google Scholar]
- Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol. 1993;329:85–110. doi: 10.1002/cne.903290107. [DOI] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–52. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lamb TD. Evolution of vertebrate retinal photoreception. Philos Trans R Soc B. 2009;364:23. doi: 10.1098/rstb.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Grünert U. Retinal connectivity and primate vision. Prog Retin Eye Res. 2010;29:622–39. doi: 10.1016/j.preteyeres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chen L, Chen M, Ye M, Seal RP, Zhou ZJ. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron. 2014;84:708–15. doi: 10.1016/j.neuron.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–54. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, DeVries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nat Neurosci. 2006;9:669–75. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- Lindstrom SH, Ryan DG, Shi J, DeVries SH. Kainate receptor subunit diversity underlying response diversity in retinal off bipolar cells. J Physiol. 2014;592:1457–77. doi: 10.1113/jphysiol.2013.265033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Weick M, Stafford BK, Demb JB. NMDA receptor contributions to visual contrast coding. Neuron. 2010;67:280–93. doi: 10.1016/j.neuron.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manu M, Baccus SA. Disinhibitory gating of reitnal output by transmission from an amacrine cell. PNAS. 2011;108(45):18447–52. doi: 10.1073/pnas.1107994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Anderson JR, Sigulinsky C, Lauritzen S. Retinal connectomics: towards complete, accurate networks. Prog Retin Eye Res. 2013;37:141–62. doi: 10.1016/j.preteyeres.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martemyanov KA. G protein signaling in the retina and beyond: the Cogan lecture. Investig Ophthalmol Vis Sci. 2014;55:8201–7. doi: 10.1167/iovs.14-15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–70. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–80. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–49. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Mills SL, Tian LM, Hoshi H, Whitaker CM, Massey SC. Three distinct blue-green color pathways in a mammalian retina. J Neurosci. 2014;34:1760–68. doi: 10.1523/JNEUROSCI.3901-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. PNAS. 2009;106:19174–78. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Kolb H. A17: A broad-field amacrine cell in the rod system of the cat retina. J Neurophysiol. 1985;54:592–614. doi: 10.1152/jn.1985.54.3.592. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, Leung KM, Odermatt B, Lagnado L. Synaptic mechanisms of adaptation and sensitization in the retina. Nat Neurosci. 2013;16:934–41. doi: 10.1038/nn.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photore-ceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–74. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Nikolaev A, Lagnado L. Encoding of luminance and contrast by linear and nonlinear synapses in the retina. Neuron. 2012;73:758–73. doi: 10.1016/j.neuron.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005;47:739–50. doi: 10.1016/j.neuron.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci. 2011;14:1555–61. doi: 10.1038/nn.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa H, Hoon M, Yoshimatsu T, Della Santina L, Wong RO. Illuminating the multifaceted roles of neurotransmission in shaping neuronal circuitry. Neuron. 2014;83:1303–18. doi: 10.1016/j.neuron.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Hartveit E. Transient release kinetics of rod bipolar cells revealed by capacitance measurement of exocytosis from axon terminals in rat retinal slices. J Physiol. 2010;588:1469–87. doi: 10.1113/jphysiol.2010.186916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–8. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- Ozuysal Y, Baccus SA. Linking the computational structure of variance adaptation to biophysical mechanisms. Neuron. 2012;73:1002–15. doi: 10.1016/j.neuron.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer OS, Verweij J, Li PH, Schnapf JL, Dacey DM. Blue-yellow opponency in primate S cone photoreceptors. J Neurosci. 2010;30:568–72. doi: 10.1523/JNEUROSCI.4738-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Paul DL, Bloomfield SA, Volgyi B. Connexin36 is required for gap junctional coupling of most ganglion cell subtypes in the mouse retina. J Comp Neurol. 2010;518:911–27. doi: 10.1002/cne.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJH, Kim I-J, Looger LL, Demb JB, Borghuis BG. Excitatory synaptic inputs to mouse On-Off direction-selective retinal ganglion cells lack direction tuning. J Neurosci. 2014;34:3976–81. doi: 10.1523/JNEUROSCI.5017-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJH, Borghuis BG, Rahamani P, Zeng Q, Kim IJ, Demb JB. Function and circuitry of VIP+ interneurons in the mouse retina. J Neurosci. 2015;35:10685–700. doi: 10.1523/JNEUROSCI.0222-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JT, Kerschensteiner Ambient illuminantion switches contrast preference of specific retinal processing streams. J Neurophysiol. 2015;114:540–50. doi: 10.1152/jn.00360.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. Imperfect space clamp permits electrotonic interactions between inhibitory and excitatory synaptic conductances, distorting voltage clamp recordings. PLOS ONE. 2011;6:e19463. doi: 10.1371/journal.pone.0019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, von Gersdorff H. Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron. 2000;25:215–27. doi: 10.1016/s0896-6273(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Puller C, Haverkamp S, Neitz M, Neitz J. Synaptic elements for GABAergic feed-forward signaling between HII horizontal cells and blue cone bipolar cells are enriched beneath primate S-cones. PLOS ONE. 2014;9:e88963. doi: 10.1371/journal.pone.0088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthussery T, Percival KA, Venkataramani S, Gayet-Primo J, Grunert U, Taylor WR. Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. J Neurosci. 2014;34:7611–21. doi: 10.1523/JNEUROSCI.4855-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinagel P, Zador AM. Natural scene statistics at the centre of gaze. Network. 1999;10:341–50. [PubMed] [Google Scholar]
- Rivlin-Etzion M, Wei W, Feller MB. Visual stimulation reverses the directional preference of direction-selective retinal ganglion cells. Neuron. 2012;76:518–25. doi: 10.1016/j.neuron.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–87. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Russell TL, Werblin FS. Retinal synaptic pathways underlying the response of the rabbit local edge detector. J Neurophysiol. 2010;103:2757–69. doi: 10.1152/jn.00987.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci. 2015;38:221–46. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- Saszik S, DeVries SH. A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J Neurosci. 2012;32:297–307. doi: 10.1523/JNEUROSCI.2739-08.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J Neurosci. 1999;19:1203–16. doi: 10.1523/JNEUROSCI.19-04-01203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G, Rieke F. Perspectives on: information and coding in mammalian sensory physiology: nonlinear spatial encoding by retinal ganglion cells: when 1 + 1 ≠ 2. J Gen Physiol. 2011;138(3):283–90. doi: 10.1085/jgp.201110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, et al. The spatial structure of a nonlinear receptive field. Nat Neurosci. 2012;15:1572–80. doi: 10.1038/nn.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung HS, Sbümbül U. Neuronal cell types and connectivity: lessons from the retina. Neuron. 2014;83:1262–72. doi: 10.1016/j.neuron.2014.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Enroth-Cugell C. Visual adaptation and retinal gain controls. Prog Retin Res. 1984;3:263–346. [Google Scholar]
- Sher A, DeVries SH. A non-canonical pathway for mammalian blue-green color vision. Nat Neurosci. 2012;15:952–53. doi: 10.1038/nn.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–33. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–98. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Sivyer B, Williams SR. Direction selectivity is computed by active dendritic integration in retinal ganglion cells. Nat Neurosci. 2013;16:1848–56. doi: 10.1038/nn.3565. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Kohn A. Moving sensory adaptation beyond suppressive effects in single neurons. Curr Biol. 2014;24:R1012–22. doi: 10.1016/j.cub.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford BK, Manookin MB, Singer JH, Demb JB. NMDA and AMPA receptors contribute similarly to temporal processing in mammalian retinal ganglion cells. J Physiol. 2014;592:4877–89. doi: 10.1113/jphysiol.2014.276543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikra T, Trenholm S, Drinnenberg A, Juttner J, Raics Z, et al. Rods in daylight act as relay cells for cone-driven horizontal cell-mediated surround inhibition. Nat Neurosci. 2014;17:1728–35. doi: 10.1038/nn.3852. [DOI] [PubMed] [Google Scholar]
- Szmajda BA, Devries SH. Glutamate spillover between mammalian cone photoreceptors. J Neurosci. 2011;31:13431–41. doi: 10.1523/JNEUROSCI.2105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012;31:407–41. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikidji-Hamburyan A, Reinhard K, Seitter H, Hovhannisyan A, Procyk CA, et al. Retinal output changes qualitatively with every change in ambient illuminance. Nat Neurosci. 2015;18:66–74. doi: 10.1038/nn.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Turner MH, Smith RG, et al. Nonlinear dendritic integration of electrical and chemical synaptic inputs drives fine-scale correlations. Nat Neurosci. 2014;17:1759–66. doi: 10.1038/nn.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Schwab DJ, Balasubramanian V, Awatramani GB. Lag normalization in an electrically coupled neural network. Nat Neurosci. 2013;16:154–56. doi: 10.1038/nn.3308. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci. 2012;13:194–208. doi: 10.1038/nrn3165. [DOI] [PubMed] [Google Scholar]
- Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. J Neurosci. 2010;30:15664–76. doi: 10.1523/JNEUROSCI.2081-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani S, Van Wyk M, Buldyrev I, Sivyer B, Vaney DI, Taylor WR. Distinct roles for inhibition in spatial and temporal tuning of local edge detectors in the rabbit retina. PLOS ONE. 2014;9:e88560. doi: 10.1371/journal.pone.0088560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci. 2006;9:1388–96. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Vlasits AL, Bos R, Morrie RD, Fortuny C, Flannery JG, et al. Visual stimulation switches the polarity of excitatory input to starburst amacrine cells. Neuron. 2014;83:1172–84. doi: 10.1016/j.neuron.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–88. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- Vroman R, Klaassen LJ, Howlett MH, Cenedese V, Klooster J, et al. Extracellular ATP hydrolysis inhibits synaptic transmission by increasing pH buffering in the synaptic cleft. PLOS Biol. 2014;12:e1001864. doi: 10.1371/journal.pbio.1001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TM, Holzhausen LC, Kramer RH. Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat Neurosci. 2014;17:262–68. doi: 10.1038/nn.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci. 2011;31:7670–81. doi: 10.1523/JNEUROSCI.0629-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–17. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick M, Demb JB. Delayed-rectifier K channels contribute to contrast adaptation in mammalian retinal ganglion cells. Neuron. 2011;71:166–79. doi: 10.1016/j.neuron.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Pottek M, He S, Vaney DI. Modulation of coupling between retinal horizontal cells by retinoic acid and endogenous dopamine. Brain Res Brain Res Rev. 2000;32(1):121–29. doi: 10.1016/s0165-0173(99)00071-5. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR. Imaging single cells in the living retina. Vis Res. 2011;51:1379–96. doi: 10.1016/j.visres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J Neurosci. 2006;26:12351–61. doi: 10.1523/JNEUROSCI.1071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Physiology and morphology of color-opponent ganglion cells in a retina expressing a dual gradient of S and M opsins. J Neurosci. 2009;29:2706–24. doi: 10.1523/JNEUROSCI.5471-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Farrow K, Ghanem A, Hillier D, Balint K, et al. The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron. 2013;79:1078–85. doi: 10.1016/j.neuron.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Manookin MB, Borghuis BG, Boahen K, Demb JB. Functional circuitry for peripheral suppression in mammalian Y-type retinal ganglion cells. J Neurophysiol. 2007;97:4327–40. doi: 10.1152/jn.01091.2006. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–97. doi: 10.1523/JNEUROSCI.4984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. PNAS. 2012;109:E2391–98. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McCall MA. Receptor targets of amacrine cells. Vis Neurosci. 2012;29(1):11–29. doi: 10.1017/S0952523812000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZY, Wan QF, Thakur P, Heidelberger R. Capacitance measurements in the mouse rod bipolar cell identify a pool of releasable synaptic vesicles. J Neurophysiol. 2006;96:2539–48. doi: 10.1152/jn.00688.2006. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Hauswirth WW, DeVries SH. Genetically targeted binary labeling of retinal neurons. J Neurosci. 2014;34:7845–61. doi: 10.1523/JNEUROSCI.2960-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]