Abstract
Background
Frail lung transplant candidates are more likely to be delisted or die without receiving a transplant. Further knowledge of what frailty represents in this population will assist in developing interventions to prevent frailty from developing. We set out to determine whether frail lung transplant candidates have reduced exercise capacity independent of disease severity and diagnosis.
Methods
Sixty-eight adult lung transplant candidates underwent cardiopulmonary exercise testing (CPET) and a frailty assessment (Fried’s Frailty Phenotype (FFP)). Primary outcomes were peak workload and peak aerobic capacity ( ). We used linear regression to adjust for age, gender, diagnosis, and lung allocation score (LAS).
Results
The mean ± SD age was 57 ± 11 years, 51% were women, 57% had interstitial lung disease, 32% had chronic obstructive pulmonary disease, 11% had cystic fibrosis, and the mean LAS was 40.2 (range 19.2 to 94.5). In adjusted models, peak workload decreased by 10 watts (95% CI 4.7 to 14.6) and peak decreased by 1.8 ml/kg/min (95% CI 0.6 to 2.9) per 1 unit increment in FFP score. After adjustment, exercise tolerance was 38 watts lower (95% CI 18.4 to 58.1) and peak was 8.5 ml/kg/min lower (95% CI 3.3 to 13.7) among frail participants compared to non-frail participants. Frailty accounted for 16% of the variance (R2) of watts and 19% of the variance of in adjusted models.
Conclusion
Frailty contributes to reduced exercise capacity among lung transplant candidates independent of disease severity.
Keywords: Lung transplant, frailty, peak VO2, cardiopulmonary exercise capacity, fibrosis
INTRODUCTION
Lung transplantation is widely considered to be an effective treatment for chronic respiratory failure, yet the vast majority of those affected by advanced lung disease are deemed ineligible for transplantation based on their perceived risk for serious complications following transplantation. Reduced exercise capacity and “poor functional status” have long been considered to be contraindications to transplantation, since physical stamina is required to tolerate transplant surgery and thrive despite post-operative complications1. The requirement for physical “fitness” is a challenge for many, since advancing disease severity greatly limits exercise capacity, definitions for fitness in this population are lacking, and advanced lung disease impedes the ability to maintain one’s functional status.
Recently, frailty, defined conceptually as a physical vulnerability to stressors, has risen to attention as an important phenotype in lung transplant candidates. Frail lung transplant candidates are almost twice as likely to be delisted or die without receiving a transplant2. Frailty using the Fried frailty phenotype3, is measured on a 0–5 scale with 5 being the frailest and encompasses measures of muscle strength, daily activity levels, and fatigue3, and therefore may represent an objective measure of “fitness” for surgery. Yet its relationship to maximal exercise capacity, a metric used by transplant centers to determine candidacy, remains unknown. It is possible that lower exercise capacity in lung transplant candidates can be largely explained by greater disease severity. Alternatively, frailty may capture unique information impacting exercise capacity that is independent of disease severity, a finding which would have important consequences for transplant candidacy decisions. Therefore, we hypothesized that frailty in lung transplant candidates would be associated with reduced exercise capacity, independent of disease severity and other confounding factors of exercise capacity.
We tested whether frailty was associated with reduced peak aerobic capacity ( peak) and peak workload during cardiopulmonary exercise testing in adults with advanced lung disease undergoing lung transplant evaluation4, while controlling for disease severity. We also examined whether frailty was associated with a number of other measures of exercise performance found to be predictive of reduced exercise capacity and/or poor surgical outcomes in those with pulmonary disease, including: oxygen economy ( /Work rate slope), heart rate-oxygen uptake relationship (HR/ slope), reduced breathing reserve, minute ventilation ( ), oxygen saturation (SpO2), ventilatory equivalent for carbon dioxide slope ( / slope), end tidal CO2 (ETCO2 mmHg), heart rate reserve (HRR) and systolic blood pressure (SBP)5–9.
Materials and Methods
Study Design, Participants, and Setting
We conducted a single center cross-sectional study of adults undergoing outpatient evaluation for lung transplantation at Columbia University Medical Center between December 22, 2010 and September 24, 2015, who were enrolled in the Lung Transplant Body Composition Study (LTBC)2,10–12 (Figure 1). CPET within CPET was performed as a standard clinical assessment for lung transplant evaluation. Analysis of the CPET data was performed post hoc to the original study. Inclusion criteria was enrollment in the LTBC study. Exclusion criteria for the study was a lack of a CPET within 3 months of the participant’s frailty assessment. All participants provided informed consent for participation and the Columbia University Medical Center Institutional Review Board approved the study (IRB protocol #AAAI1000).
Figure 1. Study Population Flow Diagram.
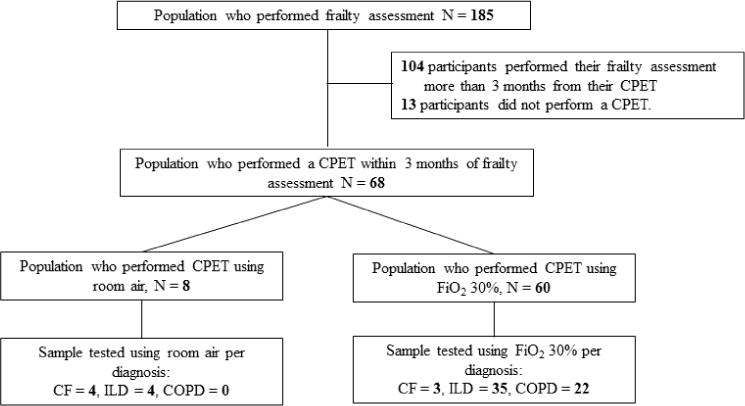
COPD: FFP; Fried Frailty Phenotype, COPD; Chronic Obstructive Pulmonary Disease, CF; Cystic Fibrosis, ILD; Interstitial Lung Disease.
Measurement of Frailty
The primary exposure of interest was the 5-point Fried Frailty Phenotype score (FFP)3. Briefly, the FFP is an aggregated score that consists of five components: shrinking (> 10 lb. unintentional weight loss in the past year), muscle weakness (grip strength measured by dynamometer), exhaustion (using two questions from the Center for Epidemiological Studies Depression scale (CESD)13), slowness (time to walk 4.57 m), and low physical activity level (< 270 Kcals for women and <383 Kcal for men expended per week based on the Minnesota Leisure Time Activity questionnaire14). Each of the 5 components is scored as “frail” or “not frail” based on established criteria3. The FFP is calculated by summing the total number of components scored as frail, with a range of 0 to 5. To achieve an adequate sample size a window of 3 months between tests was allotted.
Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing (CPET) testing is a cardiac stress test that also measures gas exchange and ventilatory parameters, used to determine the primary limitation to exercise, peak, and peak aerobic power output (workload/watts)4,15. The primary outcomes of interest were peak workload (watts, % predicted) and peak oxygen utilization ( , ml/kg/min and % predicted), obtained by a symptom-limited CPET testing using a Vmax Encore 29 metabolic cart and Viasprint 2900 cycle ergometer (Carefusion, Palm Spring, CA 92887). Secondary measures of interest were: ETCO2 mmHg, SpO2%, HRR, SBP, / slope, HR/ slope and /work rate slope. Data from the last 20 seconds of the ramped exercise phase were considered “peak”. The slope, HR/ slope and /work rate slope were measured from the onset of the ramping exercise phase and ending at the last data point before recovery. HRR was calculated by determining the change in HR from rest to peak exercise divided by the difference of the resting HR and the age predicted maximum HR (220-age)16. Ramping protocol was either a 5-watt incremental ramp if maximal voluntary ventilation (MVV) was <40 L/min or 10-watt incremental ramp if MVV ≥ 40 L/min. Similarly to prior research in this patient population17, participants were tested on FiO2 of 30% if they had been previously prescribed supplemental oxygen with exercise or had a resting oxygen saturation ≤ 90% (Figure 1).
Quantification of Disease Severity
The lung allocation score (LAS) is an excellent measure of disease severity and the risk of death across advanced lung diseases18,19. Components of the LAS are: diagnosis, age, bilirubin, BMI, cardiac index, central venous pressure, continuous mechanical ventilation, creatinine, diabetes, diagnosis, forced vital capacity (FVC), functional status, oxygen use at rest, pCO2, systolic pulmonary artery pressure, six-minute walk distance.
Pulmonary function data, including the percent predicted for FVC, forced expiratory flow one-second (FEV1) and single breath diffusion capacity for carbon monoxide (DLCO) were also recorded.
Medication Use
Glucocorticoid steroid, beta-blockade, and calcium channel blocker are medications that may impact exercise performance and can be seen in our study population. Glucocorticoids have been found to induce muscle atrophy and alter muscle function20. Beta-blockade has been found to decrease maximal exercise capacity21. Calcium channel blockers have been found to improve exercise performance22. Because of the possible influence these medications have on exercise, their use was recorded.
Analysis Approach
Continuous variables were expressed as means and standard deviation. Categorical variables were summarized by frequency and percentage. One participant was able to perform all of the testing but chose not to perform the 4.57-meter walk test and therefore the slowness component of the frailty score was imputed as a 0; imputation of a “0” was based on the practices of prior work2.
Frailty was examined both as a continuous variable (0–5) and as a categorical (not frail, intermediate frail, frail). Based on prior work by Makary et al.23 not frail was defined by a FFP score of 0–1, intermediate frail was defined by a score of 2–3 and frail was a score of ≥4.
Unadjusted associations between frailty score and exercise outcomes were tested using Spearman correlation coefficients. We used linear regression to examine associations between the FFP score (both as an ordinal continuous variable and categorized as described above) and both peak work rate and peak with adjustment for age, gender, diagnosis, and LAS. There were no missing covariate data. Assumptions of linearity were tested and met.
With alpha = 0.05, and assuming a 10% prevalence of frailty, we had 80% power to detect a difference in each measure of exercise capacity of 1.3 standard deviation units between frail and not-frail.
Analysis was performed using statistical software packages SPSS v. 24 and SAS v. 9.4. A priori α was set at 0.05.
Results
Table 1 describes the participant characteristics. One-hundred and seventy-two of the 185 candidates who performed a frailty assessment also performed a CPET. Of the 172, 68 participants performed the CPET within 3 months of their frailty assessment (Figure 1) with the average time between tests being 6 weeks (table 1). A comparison between the participants excluded and the participants included can be found in appendix table A6. People who performed their CPET outside of the 3-month window were significantly younger than those who completed a CPET within 3 months of their frailty assessment (52 ± 14 yrs vs. 57 ± 11 yrs, p=0.007). There were no other significant differences between the populations. Results of the 68 participants demonstrated three participants (4%) to have a FFP score of zero, 17 (25%) to have a score of one, 17 (25%) to have a score of two, 23 (34%) to have a score of three, 8 (12%) to have a score of four, and none had a score of five. Frail participants tended to be female, had slightly lower body mass indexes (BMI), and more commonly had chronic obstructed pulmonary disease (COPD), than the non-frail participants (Table 1).
Table 1.
Demographic, baseline clinical characteristics and exercise outcomes
| Variable | Total | Fried Frailty Phenotype Score
|
||
|---|---|---|---|---|
| Not frail (score 0–1) |
Intermediate (Score 2–3) |
Frail (Score 4) |
||
| No. of participants (% of total) | 68 | 20 (29) | 40 (59) | 8 (12) |
| Weeks between CPET and frailty score | 6 ± 5 | 6 ± 6 | 5 ± 5 | 6 ± 5 |
| Female, n (%) | 35 (51%) | 5 (25%) | 25(63%) | 5 (63%) |
| Age, years | 57 ± 11 | 57 ± 12 | 55 ± 11 | 58 ± 11 |
| Body mass index, kg/m2 | 25.4 ± 5.0 | 26.5 ± 4.1 | 25.3 ± 5.4 | 22.5± 4.3 |
| Race, n (%) | ||||
| Caucasian | 54 (79%) | 18 (90%) | 31 (77%) | 5 (63%) |
| Black | 5 (7%) | 0 | 3 (8%) | 2 (25%) |
| Hispanic | 4 (6%) | 0 | 3 (8%) | 1 (13%) |
| Asian | 5 (7%) | 2 (10%) | 3 (8%) | 0 |
| Diagnosis, n (%) | ||||
| COPD | 22 (32%) | 3 (15%) | 14 (35%) | 5 (63%) |
| CF | 7 (11%) | 3 (15%) | 3 (8%) | 1 (13%) |
| ILD | 39 (57%) | 14 (70%) | 23 (58%) | 2 (25%) |
| LAS | 40.2 ± 13.5 | 39.6 ± 13.6 | 40.7 ± 14.2 | 39.1 ± 10.2 |
| FVC % predicted | 53 ± 16 | 58 ± 18 | 51 ± 15 | 55 ± 17 |
| FEV1 % predicted | 42 ± 21 | 54 ± 21 | 38 ± 17 | 42 ± 21 |
| DLCO % predicted* | 30 ± 15 | 35 ± 12 | 28 ± 17 | 25 ± 14 |
| Medications, n (%) | ||||
| Glucocorticoid steroid | 31 (46%) | 8 (40%) | 19 (48%) | 4 (50%) |
| Beta - blockade | 8 (12%) | 2 (10%) | 4 (10%) | 2 (25%) |
| Calcium channel blocker | 4 (6%) | 0 | 3 (7%) | 1 (13%) |
Data are mean ± SD or number (%), FFP: Fried Frailty Phenotype, COPD: Chronic Obstructive Pulmonary Disease, CF; Cystic Fibrosis, ILD; Interstitial Lung Disease, LAS; Lung Allocation Score, FVC: forced vital capacity; FEV1: Forced expiratory flow 1 second; DLCO, single breath diffusion capacity for carbon monoxide.
Sample size for DLCO was 62. Three participants could not perform an adequate DLCO maneuver and three participants had missing DLCO from their charts.
Figures 2 and 3 show the distribution of peak workload and peak by FFP score (p for trend across groups <0.001 for workload and <0.001 for peak). Visual inspection of these figures suggests a threshold effect at a frailty score of 2, with similar exercise variable distributions among those scores of 0 and 1, and similar distributions among those with scores of 2 to 4 (Figures 2 and 3).
Figure 2. Distribution of peak workload by Fried Frailty Phenotype score.
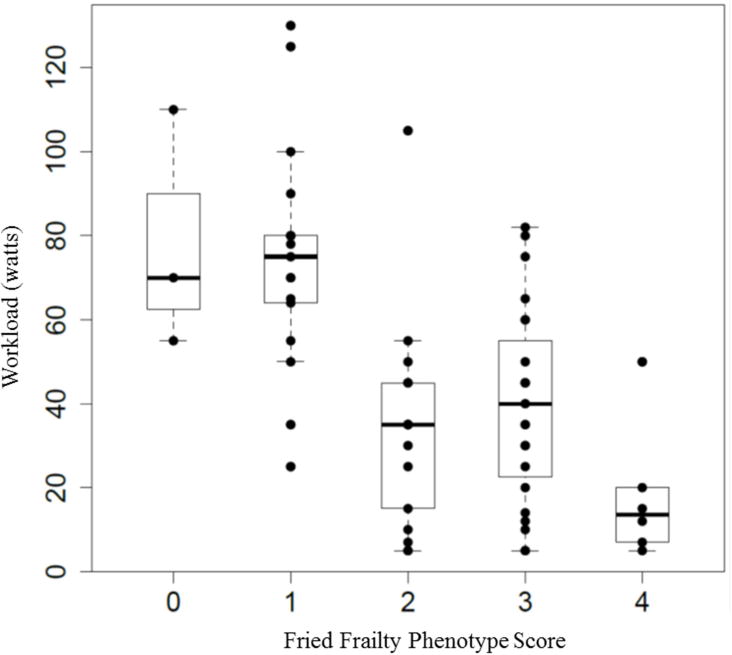
Figure 2 demonstrates the distribution of peak workload (y axis) by each frailty score (x axis).
Figure 3. Distribution of peak by Fried Frailty Phenotype score.
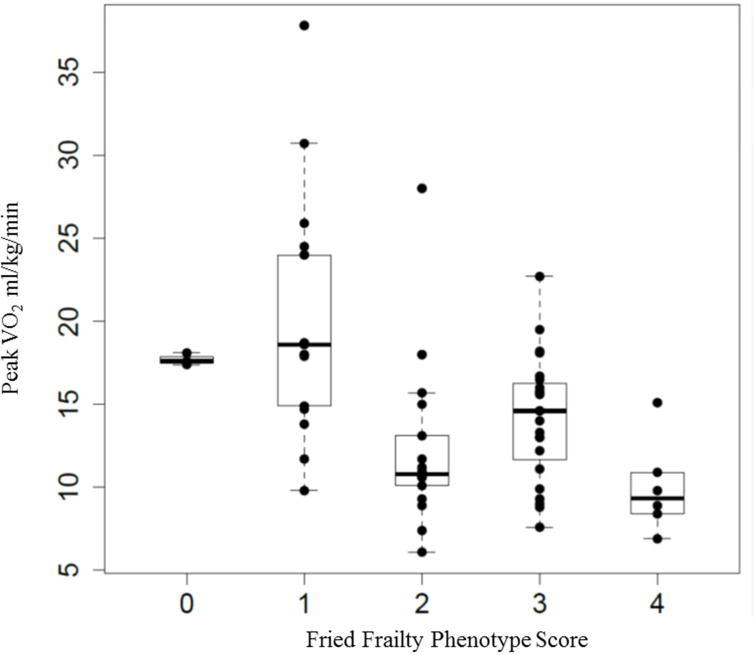
Figure 2 demonstrates the distribution of peak aerobic capacity ( peak) (y axis) by each frailty score (x axis).
Exercise performance by frailty status is described in Table 2. Greater frailty was associated with lower peak workload (p for trend ≤0.001) and a lower peak (p for trend ≤0.001). The mean workload was 19 Watts among frail participants compared to 75 among non-frail (mean difference −56 W, 95% CI −77 to −35 W, p = 0.001), and the mean peak was 9.9 mL/kg/min among frail participants compared to 19.7 ml/kg/min among non-frail (mean difference −9.8 mL/kg/min, 95% CI −15 to −5 mL/kg/min, p = 0.003). Notably, while the mean % predicted / slope was slightly higher among frail participants (123% vs 115%, p = 0.15), there was no significant trend across groups (p for trend =0.95), suggesting that frailty was not associated with a meaningfully greater ventilatory inefficiency.
Table 2.
Exercise performance during cardiopulmonary exercise testing by frailty group
| Variable | Fried Frailty Phenotype
|
p for trend | ||
|---|---|---|---|---|
| Not frail (score 0–1) |
Intermediate (Score 2–3) |
Frail (Score 4) |
||
| Workload,Watts | 75 ± 27 | 38 ± 23 | 19 ± 17 | <0.001 |
| Workload % predicted | 47 ±18 | 31± 18 | 14 ± 11 | 0.002 |
| , mL/kg/min | 19.7 ± 6.6 | 13.4 ± 4.1 | 9.9 ± 2.4 | <0.001 |
| % predicted, % | 70 ± 19 | 49 ± 14 | 35 ± 7 | <0.001 |
| /Work rate slope, % predicted† | 133 ± 36 | 139 ± 66 | 190 ± 124 | 0.54 |
| SpO2,% | 92 ± 4 | 92 ± 6 | 94 ± 5 | 0.93 |
| / slope, % predicted | 115 ± 44 | 113 ± 44 | 123 ± 74 | 0.95 |
| End tidal CO2,mmHg/min | 38.0 ± 8.9 | 38.7 ± 9.7 | 38.4 ± 9.5 | 0.88 |
| Breathing reserve, % | 32 ± 4 | 22 ± 3 | 19 ± 7 | 0.04 |
| , L/min | 60.0 ± 18 | 34.5 ± 16 | 27.3 ± 18 | <0.001 |
| , % predicted | 63 ± 25 | 54 ± 19 | 51 ± 27 | 0.051 |
| SBP, mmHg | 165 ± 20 | 160 ± 18 | 143 ± 17 | 0.12 |
| Heart rate reserve % | 80 ± 9 | 71 ± 11 | 69 ± 9 | 0.001 |
| HR/ slope % predicted | 81 ± 30 | 84 ± 43 | 103 ± 50 | 0.43 |
: peak volume of oxygen utilized, /Work rate slope: increase in oxygen utilization per watt, SpO2: oxygen saturation by pulse oximetry, /V’CO2 slope: ventilatory equivalent for carbon dioxide slope, , L/min: minute ventilation, : tidal volume, SBP: Systolic Blood Pressure, HR/ slope: increase in heart rate per liter of oxygen utilized. Heart rate reserve %, a normal exercise response is to attain ≥80% of one’s heart rate reserve.
n=64, two participants could not exercise beyond the warm up period, therefore workload never increased and /work rate slope could not be obtained.
CPET parameters are an average of the last 20 seconds of peak exercise, with the exception of the /Work rate slope and / slope, which are measured from the onset of ramping to the last breath at the end of ramping.
Adjustment for age, gender, diagnosis, and lung allocation score only slightly attenuated the relationship between frailty and exercise capacity (Table 3). After adjusting for age, gender, diagnosis, and lung allocation score, the mean difference in workload between frail and non-frail was −38 W (95% CI −58 to −19 W, p ≤0.001), and mean difference in peak between frail and non-frail was −8.4 mL/kg/min (95% CI −13.7 to −3.1 mL/kg/min, p ≤0.001). In addition, each 1 point increment in FFP score (i.e. greater frailty) was associated with a 10.7 W reduction in peak workload (95% CI 5.8 to 15.6 W, p < 0.001) and with a 2.0 mL/kg/min reduction in peak (95% CI 0.9 to 3.1 mL/kg/min, p = 0.001). Frailty accounted for the greatest amount of change in variability in the adjusted model compared to other parameters. Frailty accounted for 18% of the variance (partial R2) in Watts and 20% of the variance in (Table 4).
Table 3.
Adjusted associations between FFP and exercise performance.
| Outcome | Fried Frailty Phenotype
|
p for trend | Decrement in outcome per 1 point increment in FFP score | p-value | ||
|---|---|---|---|---|---|---|
| Not frail (score 0–1) |
Intermediate (score 2–3) |
Frail (score 4) |
||||
| Watts | Ref (0) | −26.0 (−37.7 to −14.2) |
−38.1 (−57.5 to −18.7) |
<0.001 | −10.7 (−15.6 to −5.8) |
<0.001 |
| peak | Ref (0) | −5.5 (−8.1 to −2.9) |
−8.4 (−13.7 to −3.1) |
<0.001 | −2.0 (−3.1 to −0.9) |
0.001 |
| Breathing Reserve % | Ref (0) | −2.9 (−12.3 to 6.4) |
−1.0 (−11.7 to 13.8) |
0.87 | −1.0 (−4.6 to 2.5) |
0.56 |
| , L/min | Ref (0) | −10.3 (−18.0 to −2.6) |
−12.2 (−2.7 to 27.2) |
0.10 | −4.6 (−7.7 to −1.5) |
0.004 |
| Heart rate reserve % | Ref (0) | −11.1 (−5.1 to −17.16) |
−10.3 (−0.8 to −19.9) |
0.03 | −3.0 (−.4 to −5.6) |
0.02 |
Models are adjusted for age, gender, diagnosis, and lung allocation score.
Table 4.
Amount of variance within the outcome measures explained by the model
| Outcome | R2 for the model | Partial R2 for FFP | p-value* |
|---|---|---|---|
| Watts | .640 | .182 | <0.001 |
| peak | .512 | .204 | <0.001 |
Model: Dependent variable is the outcome and variables entered into the model were: age, gender, lung allocation score, diagnosis, and Fried Frailty Phenotype grouping.
Significant change in R2 with the addition of the Fried Frailty Phenotype score (FFP) into the model
Other exercise outcomes that were significantly associated with frailty were and percent heart rate reserve (Table 3). In the adjusted analyses, each 1 point increment in FFP score was associated with a reduction in of 4.6 L/min (95% CI −7.7 to −1.5 L/min, p=0.004); however that relationship was no longer significant across groups. HRR was also associated with frailty. Each 1-point increment in FFP score was associated with a decrease in HRR by −3.0% (95% CI −.4 to −5.6, p=0.02) and decrease of −10.3% (95% CI −0.5 to −19.9%, p trend 0.03) in the frail group compared to the not frail group.
Discussion
We found that frailty was statistically and clinically associated with reduced maximal exercise capacity among a cohort of lung transplant candidates at our center, independent of respiratory-disease severity and diagnosis. These finding indicate that the frailty phenotype – a resting measure – captures potentially clinically important information about physical fitness above and beyond that available from resting measures of disease severity alone in adults with advanced lung disease.
There are a number of potential explanations for our findings. The most likely explanation is that frailty is a major extra-pulmonary consequence of advanced lung disease. Potential mechanisms that are believed to contribute to frailty in the lung disease population are cachexia, chronic inflammation, disuse atrophy, muscle dysfunction and chronic hypoxia2,11. Abnormal muscle function has been noted in people with COPD and ILD24–27 and mitochondrial dysfunction has been noted in people with COPD28,29. Further exploration is needed to determine if the underlying muscle dysfunction noted in these populations is leading to frailty and reduced exercise tolerance. Future work investigating how pulmonary rehabilitation impacts frailty in this population would assist in identifying the role of deconditioning versus muscle dysfunction in the development of frailty. Previous work strongly suggests that frailty exists independently of comorbid illness and disability30. In our study, participants did not demonstrate the classic patterns consistent with a primarily cardiovascular limitation to exercise. There was no significant relationship between frailty and HR/VO2 slope (the rise in HR per liter of VO2 utilized, a measure of cardiovascular health), and excessive hypertension. The poor heart rate reserve seen in our population corresponds with prior work that reported chronotropic incompetence in both the COPD and interstitial lung disease (ILD) populations, which was associated with reduced exercise capacity31,32. Tools to measure cardiac function in the field, such as VEST33, the can be used to explore the role of cardiovascular limitations in frailty further.
The magnitudes of the differences in peak observed between frail and non-frail participants in our study were substantial. For example, current guidelines define a peak of > 15 ml/kg/min as “low risk” and a peak of <10 ml/kg/min is considered “high risk” for postoperative complications4,34. These thresholds correspond to the mean values observed in the frail and non-frail groups in our study. The similarity of our frail thresholds to these established definitions of functional capacity and inability to tolerate operative complications supports FFP as a clinically meaningful indicator of physiologic reserve.
While observational in nature, it is possible that targeting frailty (and its underlying causes and endophenotypes) using preventative or therapeutic interventions might preserve or even improve exercise capacity and even outcomes after lung transplantation. Studying the impact of interval training or resistance training versus aerobic training on oxidative stress markers, mitochondrial biogenesis, and quadriceps strength could give insight into the pathobiological abnormalities that contribute to frailty in those with lung disease and could represent surrogate outcome measures for therapeutic interventions. For example, interventions targeted at those with FFP scores of 2 or greater – a group who seem to have the lowest exercise capacity) – would be appropriate for therapeutic interventions, while preventative interventions should be targeted at those with frailty scores of 0 or 1. Future research can also investigate possible physiological changes driving the steep decline in functional capacity as individual progress from non-frail to frail.
Future work may also address the role of frailty in various phenotypes of pulmonary disease, such as the various COPD phenotypes. Recent work by Camiciottoli et al.35 described different panels of comorbidities among patients with predominant chronic bronchitis phenotype versus patients with emphysema. It is possible frailty may be more common in certain phenotypes and the mechanisms driving the presence of frailty within a diagnosis may vary. Research mapping the prevalence, mechanism and exercise response of frailty within each subpopulation would provide the fundamental knowledge needed to design an effective intervention.
Despite several strengths, our study had limitations. The greatest limitation to the study was the large percentage of the study population who had to be excluded due to the gap between visits (> 3 months). Timing between visits to the center is sometimes difficult to predict or control. To control for changes in health status between assessments, a narrow testing window was selected; however, this lead to healthier participants who take longer to complete their lung transplant evaluations and extremely ill participants who are enrolled in the transplant program as in patients, to be excluded. A strength of the frailty assessment is it can still be performed on such terminally ill patients, and a limitation of CPET. Another limitation of our study was the quantification of disease severity. We thought LAS would be a superior measure of disease severity in our paper since we have combined patients with COPD and ILD together in an analysis. For example, FEV1 would not be appropriate in the interstitial lung disease group, DLCO was missing in 9% of the population with the sickest patients unable to perform the maneuver due to insufficient vital capacity, and FVC seems inappropriate for COPD patients. Our findings may not be generalizable to those with lung disease not yet severe enough to merit referral for lung transplantation, those at transplant centers other than our own, or those who are not eligible for lung transplantation, such as those with a history of non-adherence, severe comorbidities, substance abuse, and advanced age. The laboratory that performed the testing only uses one person to administer testing. In addition, since our study was observational in nature, residual and unmeasured confounding might explain some or even all of our findings. Finally, we did not examine the mechanisms underlying the associations we observed. Future studies should focus on the associations of body composition, inflammatory markers, oxidative stress and mitochondrial dysfunction and with frailty to determine the possible mechanisms that drive exercise intolerance and muscle weakness in this population.
In summary, frailty is independently associated with reduced maximal exercise capacity among lung transplant candidates across a wide range of ages and independent of disease severity. These data provide additional construct validity for frailty as a physiologically relevant phenotype in this population, and suggest that investigators may wish to target exercise and other interventions in frail lung transplant candidates.
Supplementary Material
Highlights.
Frail lung transplant candidates have reduced exercise capacity out of proportion to the severity of their lung disease.
Frail lung transplant candidates have an average VO2 peak of less than 10 ml/kg/min
In lung transplant candidates, reduced aerobic muscle strength demonstrated the strongest correlation with frailty.
Acknowledgments
We would like to acknowledge: 1Renee Lemieux, 1Eric Peterson, 1Darnell Cain, 1Orest Paslavski for your assistance, 2Kim Stavrolakes and the physical therapy team for your support and contributions, 2Hanyoung Kim for your assistance and support, 2the lung transplant coordinators and administrative staff for making such data collection possible, 3Tatiana Blue and 3Alessandra Messineo for your assistance.
1Columbia University, New York, New York, USA; 2New York Presbyterian Hospital, New York, NY USA; 3Pulmonary & Intensive Care Translational Outcomes Research (PICTOR) Group, Columbia University Medical Center, New York, NY, USA.
Funding: This work was supported by NIH grants R01 HL114626, K24 HL131937, K23 HL111115, K23 AG045560, and the non-profit entities: Pulmonary Fibrosis Foundation, the Rocco Guinta Research Fund, and the Nina Ireland Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: There are no conflicts of interest to report.
References
- 1.Hook JL, Lederer DJ. Selecting lung transplant candidates: where do current guidelines fall short? Expert review of respiratory medicine. 2012;6(1):51–61. doi: 10.1586/ers.11.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. American journal of respiratory and critical care medicine. 2015;192(11):1325–1334. doi: 10.1164/rccm.201506-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic S, American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong HF, Schulze PC, Bacchetta M, Thirapatarapong W, Bartels MN. Impact of pulmonary hypertension on exercise performance in patients with interstitial lung disease undergoing evaluation for lung transplantation. Respirology. 2014;19(5):675–682. doi: 10.1111/resp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K. End-tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127(5):1637–1646. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 7.van der Plas MN, van Kan C, Blumenthal J, Jansen HM, Wells AU, Bresser P. Pulmonary vascular limitation to exercise and survival in idiopathic pulmonary fibrosis. Respirology. 2014;19(2):269–275. doi: 10.1111/resp.12206. [DOI] [PubMed] [Google Scholar]
- 8.Fleury B, Murciano D, Talamo C, Aubier M, Pariente R, Milic-Emili J. Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. The American review of respiratory disease. 1985;131(6):822–827. doi: 10.1164/arrd.1985.131.6.822. [DOI] [PubMed] [Google Scholar]
- 9.Wensel R, Opitz CF, Anker SD, et al. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106(3):319–324. doi: 10.1161/01.cir.0000022687.18568.2a. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin MR, Reid MC, Westlake AA, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014;29(3):401–408. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Layton AM, Armstrong HF, Baldwin MR, et al. The effect of frailty on functional capacity in lung transplant candidates. Unpublished. 2016 [Google Scholar]
- 12.Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the United States. American journal of respiratory and critical care medicine. 2014;190(9):1012–1021. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 14.Richardson MT, Leon AS, Jacobs DR, Jr, Ainsworth BE, Serfass R. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47(3):271–281. doi: 10.1016/0895-4356(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 15.Starobin D, Kramer MR, Yarmolovsky A, et al. Assessment of functional capacity in patients with chronic obstructive pulmonary disease: correlation between cardiopulmonary exercise, 6 minute walk and 15 step exercise oximetry test. The Israel Medical Association journal: IMAJ. 2006;8(7):460–463. [PubMed] [Google Scholar]
- 16.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123(9):1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawut SM, O’Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med. 2005;99(11):1431–1439. doi: 10.1016/j.rmed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Russo MJ, Iribarne A, Hong KN, et al. High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest. 2010;137(3):651–657. doi: 10.1378/chest.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, Gupta Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17(5):913–916. doi: 10.4103/2230-8210.117215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atwood JE, Sullivan M, Forbes S, et al. Effect of beta-adrenergic blockade on exercise performance in patients with chronic atrial fibrillation. J Am Coll Cardiol. 1987;10(2):314–320. doi: 10.1016/s0735-1097(87)80013-x. [DOI] [PubMed] [Google Scholar]
- 22.Pool PE, Seagren SC, Salel AF. Effects of diltiazem on serum lipids, exercise performance and blood pressure: randomized, double-blind, placebo-controlled evaluation for systemic hypertension. The American journal of cardiology. 1985;56(16):86H–91H. doi: 10.1016/0002-9149(85)90550-8. [DOI] [PubMed] [Google Scholar]
- 23.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Doucet M, Russell AP, Leger B, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2007;176(3):261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 25.Holland AE. Exercise limitation in interstitial lung disease - mechanisms, significance and therapeutic options. Chron Respir Dis. 2010;7(2):101–111. doi: 10.1177/1479972309354689. [DOI] [PubMed] [Google Scholar]
- 26.Mador MJ, Bozkanat E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Res. 2001;2(4):216–224. doi: 10.1186/rr60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiyama O, Taniguchi H, Kondoh Y, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127(6):2028–2033. doi: 10.1378/chest.127.6.2028. [DOI] [PubMed] [Google Scholar]
- 28.Puente-Maestu L, Perez-Parra J, Godoy R, et al. Abnormal transition pore kinetics and cytochrome C release in muscle mitochondria of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;40(6):746–750. doi: 10.1165/rcmb.2008-0289OC. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovich RA, Bastos R, Ardite E, et al. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J. 2007;29(4):643–650. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- 30.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland AE, Hill CJ, Glaspole I, Goh N, Dowman L, McDonald CF. Impaired chronotropic response to 6-min walk test and reduced survival in interstitial lung disease. Respir Med. 2013;107(7):1066–1072. doi: 10.1016/j.rmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Costello J, Armstrong HF, Jorde UP, et al. Chronotropic incompetence predicts mortality in severe obstructive pulmonary disease. Respir Physiol Neurobiol. 2013;188(2):113–118. doi: 10.1016/j.resp.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acanfora D, Scicchitano P, Casucci G, et al. Exercise training effects on elderly and middle-age patients with chronic heart failure after acute decompensation: A randomized, controlled trial. Int J Cardiol. 2016;225:313–323. doi: 10.1016/j.ijcard.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation. 2016;133(24):e694–711. doi: 10.1161/CIR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 35.Camiciottoli G, Bigazzi F, Magni C, et al. Prevalence of comorbidities according to predominant phenotype and severity of chronic obstructive pulmonary disease. International journal of chronic obstructive pulmonary disease. 2016;11:2229–2236. doi: 10.2147/COPD.S111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


