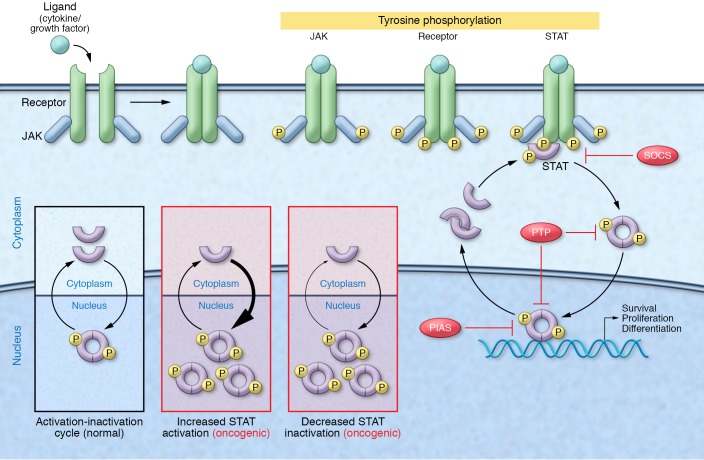
Figure 1. STAT-mediated oncogenesis.
Under physiologic conditions, STAT signaling is stimulus dependent and tightly regulated by endogenous inhibitors, including SOCS proteins, protein inhibitor of activated STAT (PIAS) proteins, and protein tyrosine phosphatases (PTPs). Cytokine or growth factor stimulation triggers receptor oligomerization and sequential tyrosine phosphorylation of receptor-associated JAKs, intracellular receptor domains, and newly recruited STAT proteins. Phosphorylated STAT dimers then translocate to the nucleus, where they bind DNA and control the expression of genes regulating proliferation, survival, and differentiation. Following dephosphorylation, STATs are shuttled back out of the nucleus, completing the activation-inactivation cycle. Cancer-associated events can lead to constitutive STAT activity and STAT-dependent oncogenesis. This can occur through increased STAT activation due to elevated cytokine levels, loss of endogenous inhibitors, or hyperactivation of upstream signaling machinery, or via decreased STAT inactivation, such as that occurring through loss of endogenous inhibitors and dephosphorylation-resistant STAT mutants. The STAT5 mutant STAT5BN642H analyzed by Pham et al. (18) appears to drive the malignant transformation of hematopoietic cells through this latter mechanism.