Figure 1.
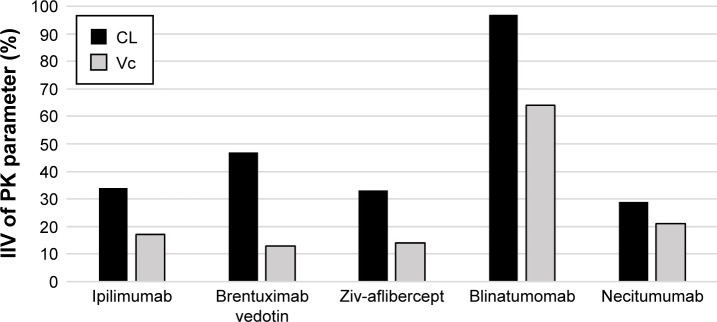
Of the 15 biologic therapeutics for oncology published between 2005 and 2016 in FDA’s Purple Book, 5 drugs had defined positive E–R relationship for both efficacy and safety.
Note: Blincyto® (blinatumomab [Amgen, Thousand Oaks, CA, USA]) had nearly 2-fold the fluctuation in serum concentration as compared to the drug, with the second highest fluctuation in serum concentration and documented safety E–R relationship suggesting significant fluctuations in serum concentrations and, therefore, increased risk of either suboptimal disease control in patients with high CL or increased risk of adverse drug reactions in patients with low CL.
Abbreviations: CL, clearance; E–R, exposure–response; FDA, US Food and Drug Administration; IIV, inter-individual variability; PK, pharmacokinetics; Vc, central volume of distribution.