Abstract
OBJECTIVE: To determine whether actinic keratosis and photodamaged perilesional areas (field cancerization) treated successfully with topical ingenol mebutate gel 0.015% remained clear one year later, and to treat actinic keratosis and perilesional skin not treated one year earlier. DESIGN: Single-center, single-arm, open-label extension of an original clinical study completed one year earlier. SETTING: An outpatient clinic. PARTICIPANTS: Fifteen of the original 28 study patients enrolled in and who completed the extension phase. MEASUREMENTS: All treated and untreated lesions in the original study were evaluated clinically, dermoscopically, and with optical coherence tomography at Day 0 of the extension study. Previously untreated lesions were then treated with ingenol mebutate gel 0.015% for three days and reevaluated at Day 60. RESULTS: There was no significant increase in actinic keratoses over one year. The majority of actinic keratoses not treated in the original study were still present at the beginning of the extension study. Following treatment, 69 percent of these lesions cleared by Day 60 of the extension study, which was not significantly different from the 79 percent clearance observed in the original study. CONCLUSION: Ingenol mebutate 0.015% maintained clearance of lesions treated one year earlier. Optical coherence therapy demonstrated its reliability as a noninvasive mode of diagnosis for actinic keratosis as well as actinic damage in the surrounding areas of field cancerization. Optical coherence therapy also showed that previously untreated lesions exhibited similar clearance rates once treated with the medication.
Keywords: Actinic keratosis, ingenol mebutate, perilesional, field cancerization, photodamage, optical coherence tomography
Actinic keratosis (AK) is a product of cumulative exposure to ultraviolet (UV) light and is considered the most common precursor of invasive squamous cell carcinoma (SCC) of the skin.1,2 The relative risk of progression from AK to SCC depends on patient-related factors, such as immune status and Fitzpatrick skin category, as well as on the presence of AK-associated features, such as lesional thickness, inflammatory changes, and the extent of photodamage to perilesional skin.3 Studies assessing the rate of progression to SCC have found considerable variability, but averaging and extrapolation of the data suggest a 10-year risk of dermal invasion of at least one lesion to be about eight percent,2 thus making AK an excellent target for preventive strategies. Visible AKs are well-accepted candidates for treatment, before these lesions have the opportunity to become more aggressive.
The skin surrounding a visible AK lesion is subject to the same UV radiation-induced damage that promotes the AK and can harbor dysplastic subclinical lesions that are genetically similar to AK but not clinically apparent.4,5 For this reason, field cancerization, which is defined as the damaging effects of chronic UV exposure on large areas of skin, increases the likelihood of the development of AKs and their potential progression into SCC.4 Field-directed therapy rather than lesion-directed therapy is warranted for multiple visible (clinical) AKs on contiguous areas, and also for suspected subclinical lesions or contiguous sun-damaged areas at risk for subclinical lesions.6 Identifying and treating field cancerization might prevent the development of additional AKs and potentially reduce the risk of future progression to SCC. Clinically visible AKs and the surrounding normal-appearing skin within the field of cancerization can be identified by biopsy4 or specialized imaging,5,7 including reflectance-mode confocal microscopy6–9 and optical coherence tomography (OCT).4,8,10–12
In an effort to utilize reliable diagnostic tools that are noninvasive and therefore might spare the patient a biopsy, we conducted a pilot study involving OCT diagnosis of patients treated with ingenol mebutate gel 0.015%. In 2016, we reported the results of this open-label, split-face study that was designed primarily to delineate actinic field cancerization by comparing OCT with conventional histopathology for the identification of clinical and subclinical AKs.4 To correlate OCT, histopathologic, and clinical diagnoses, OCT imaging followed by biopsy was used to examine clinically identified AKs and the sun-exposed perilesional skin. We found that OCT analysis had a 100-percent correlation with the clinical diagnosis of AK and also detected 79 percent of histopathologically confirmed subclinical lesions from perilesional skin sites that appeared clinically normal. The study was furthermore designed to determine the efficacy of ingenol mebutate gel 0.015% for field-directed treatment of clinical AKs and for subclinical AKs on sun-exposed perilesional skin. The results showed that ingenol mebutate effectively cleared AK, particularly clinically characteristic lesions.4
Given the statistically significant results for clearance and the diagnostic potential of OCT, we conducted the current study as an extension of the original study. Our objectives were 1) to determine whether the AKs successfully treated during the original study remained clear one year later and 2) to treat the previously untreated clinical AKs (from the untreated side of the original split-face study) and any subclinical lesions on the photodamaged perilesional skin.
METHODS
This was a single-center, single-arm, open-label extension of an original clinical study completed one year earlier. The methodology of the extension phase was similar to that of the original study, which has been reported previously.4 Briefly, in the original study, patients were required to have at least seven clinical AKs on the face in three separate areas, including three lesions on each side of the face. Patients were excluded if they had a history or evidence of skin conditions other than AK or if lesions that exhibited an atypical appearance were identified within the treatment area. In total, 30 Caucasian male patients 18 years of age or older with a past history of AK treatment were enrolled. Two of the patients did not return for follow-up and were not included in the analysis. The 28 patients who completed the original study were invited to participate in the extension study one year later. Those patients who were eligible and enrolled in the extension study provided written informed consent and allowed the clinical research staff to take photographs of the selected facial lesions.
Patients were evaluated by clinical assessment, dermoscopy, and OCT imaging (VivoSight® OCT Scanner, Michelson Diagnostics; Maidstone, Kent, United Kingdom) to track the behavior of the three previously treated AKs (on the treated side of the original split-face study), the three previously untreated AKs (on the untreated side of the split-face study), and the chronically sun-exposed skin surrounding these AKs identified in the original study. Evaluations were performed at baseline (Day 0) in an outpatient clinic setting, after which ingenol mebutate gel 0.015% was applied by the patient at home once daily for three consecutive days (Days 1, 2, and 3) to the area of the face containing three previously untreated AKs and the perilesional skin. Clinical assessment was repeated, and the lesions were photographed after the third application of ingenol mebutate on Day 3. Patients returned on Day 60 for clinical reassessment and repeat dermoscopy and OCT imaging. Lesions were classified as (typical) AK, hypertrophic AK, or SCC in situ (Bowen's disease; bowenoid AK), based mainly on OCT criteria but also on clinical appearance, when visible, and histopathology, when available. The photographs of clinical lesions also were used to assess local skin responses (LSRs) as none, mild, moderate, or severe.
Statistical analysis was performed using the paired t-test, chi-square test, and Fisher's exact test to determine statistical significance. For purposes of statistical analysis, we also classified lesions as clinical (visible) lesions (AK + non-AK), subclinical lesions (subclinical AK + subclinical non-AK), and total lesions (clinical + subclinical lesions).
RESULTS
Of the 30 patients enrolled in the original study, 15 of them subsequently participated in the extension phase. Of the 15 nonparticipant patients from the original study, two did not return for follow-up during the original study (see above), eight declined to participate, four could not be contacted, and one was denied entry due to a medical comorbidity.
Facial areas that were treated during the original study primarily maintained their clearance during the extension study. In the original study, there was a statistically significant reduction (P<0.00001) in the average number of clinical lesions per patient from Day 0 to Day 60 following treatment (Figure 1A). At one year later, on Day 0 of the extension study, clinical lesion counts remained low in those patients enrolled in the extension study (Figure 1B). Thus, there was no statistically significant difference between the number of lesions (AK and subclinical AK) present at the end of the original study and the number of lesions present at the beginning of the extension study for both treated (P<0.262) and untreated groups (P<0.959) (Figures 2A and 2B).
FIGURE 1.
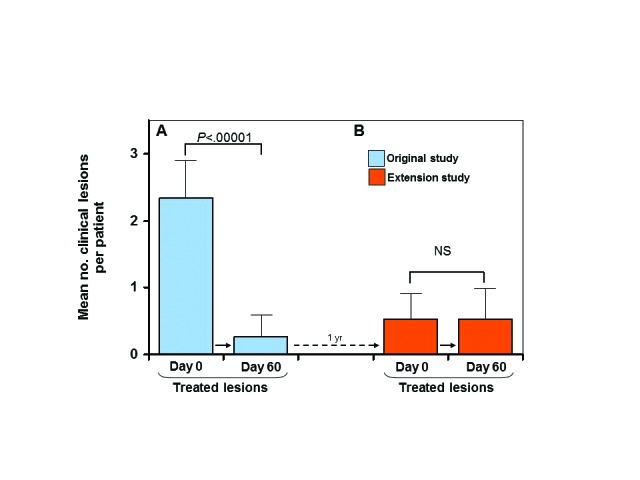
Previously treated lesions—mean number of clinical lesions per patient between Day 0 and Day 60 of the original study in the treated group (A); mean number of clinical lesions between Day 0 and Day 60 in the extension study (all lesions were treated on Day 0) (B); solid arrows indicate transition from Day 0 to Day 60 for each study; dotted arrow indicates 1-year transition from Day 60 of original study to Day 0 of extension study; error bars=95% confidence interval; NS=not significant
FIGURE 2.
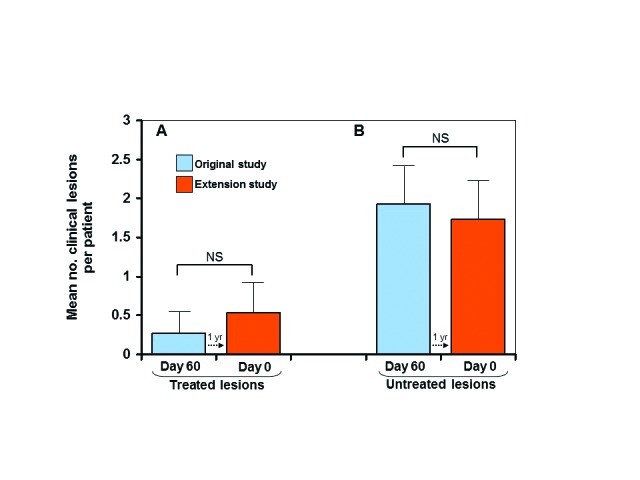
Maintenance of clearance—mean number of clinical lesions per patient at 60 days after treatment in the original study vs Day 0 of the extension study (A); mean number of clinical lesions per patient at 60 days after no treatment in the original study vs Day 0 of the extension study (B); dotted arrows indicate 1 -year transition from Day 60 of the original study to Day 0 of the extension study; error bars=95% confidence interval; NS=not significant
Clearance rates of clinical and subclinical lesions from the original split-face untreated side that were subsequently treated in this extension study were comparable to rates achieved in the original study. In the original study, 79 percent of the 92 clinical and subclinical lesions (including hypertrophic AKs and SCC in situ, as well as typical AKs) diagnosed by OCT were cleared at Day 60 after treatment. In contrast, only 17 percent of the 69 untreated clinical and subclinical lesions diagnosed by OCT were spontaneously clear at Day 60. These untreated lesions were subsequently treated in the extension study and exhibited 69 percent clearance. This difference in clearance rate (17% vs. 69%) was also statistically significant (P<0.0001 ) (Figure 3) and demonstrates the efficacy of the treatment. Finally, when comparing the original study with this extension study, the overall difference in clearance rates of all clinical and subclinical lesions (79% vs 69%) that received treatment was not statistically significant (P=0.2336) (Figure 4) and again emphasizes that the medication was effective in treating field cancerization.
FIGURE 3.
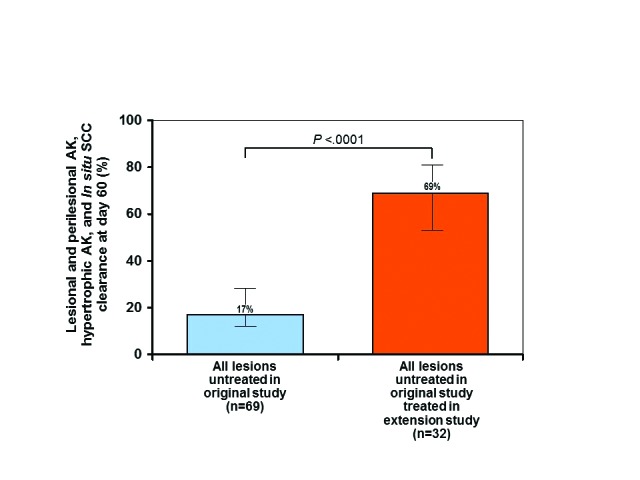
Clearance of all untreated clinical and subclinical lesions in the original study after treatment in the extension study—error bars=95% confidence interval. *actinic keratosis (AK), hypertrophic AK, squamous cell carcinoma (SCC) in situ (Bowen's disease; bowenoid AK)
FIGURE 4.
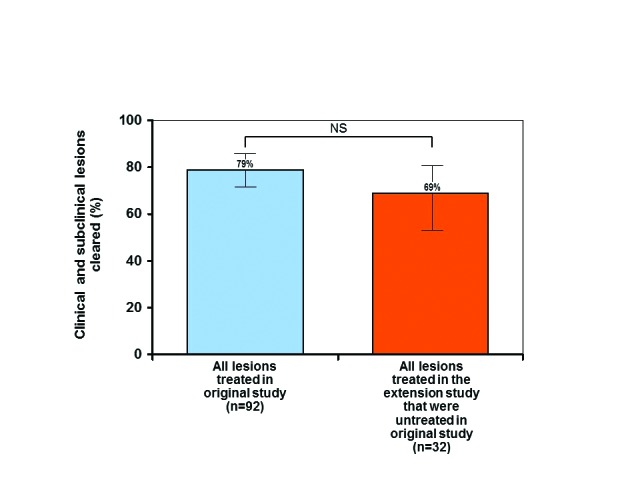
Clearance of all treated clinical and subclinical lesions after treatment in the original and extension studies—error bars=95% confidence interval; *actinic keratosis ( hypertrophic AK, squamous cell carcinoma (SCC) in situ (Bowen's disease; bowenoid AK)
FIGURE 5.
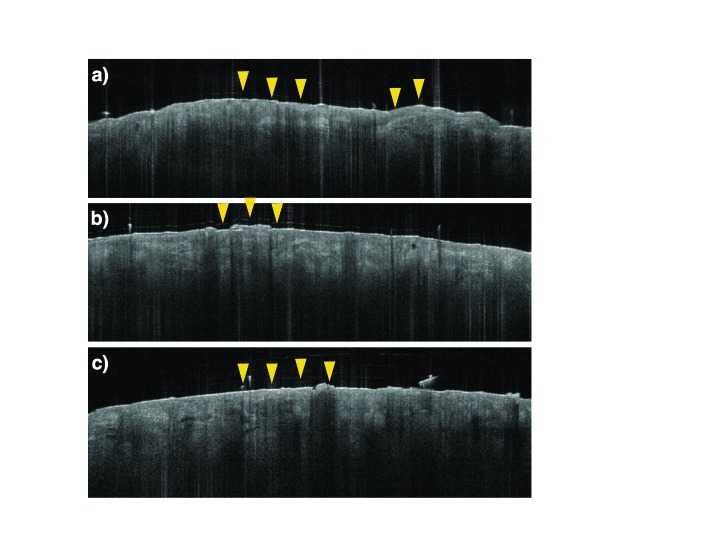
Optical coherence tomography (OCT) images of nonclearing actinic keratosis (AKs)—(A) prior to treatment, (B) following treatment (Day 60 of the original study), and (C) when evaluated at the start of the extension study 12 months later; during all stages, AKs featured characteristics seborrheic keratoses, with areas of thickened and very well-defined architectural plateaus (yellow arrows)
LSRs were assessed as mild in 12 patients, moderate in two patients, and severe in one patient. There was no statistically significant correlation between LSR severity and clearance.
DISCUSSION
Our original study, published in 2016, was innovative in that we correlated noninvasive imaging with histopathology and revealed the concept of field cancerization beyond what can be visualized clinically. After performing biopsies on areas that appeared normal (“subclinical lesions”), we found that 79 percent of these normal-appearing areas actually contained actinic damage, which was clearly diagnosed on OCT. This finding demonstrated the importance of noninvasive imaging in diagnosing early precancerous lesions that would otherwise go unnoticed. Based on the high correlation between histopathologic and OCT diagnoses (100% for clinical lesions and 79% for subclinical lesions), we were able to monitor reliably the response of clinical and subclinical lesions that were either treated with ingenol mebutate 0.015% or left untreated. Considering that some AKs can be refractory to cryotherapy,13 the findings in our original study prompted us to pursue an extension study to assess the durability of clearance after one year and offer patients the benefit of clearing their originally untreated lesions.
In addition to knowing that they can treat AKs effectively, it is also important to clinicians to know that lesion clearance can be maintained long term. Among treated lesions, we found that there was no statistically significant difference in the average number of treated clinical and subclinical lesions between the end of the original study and the beginning of the extension study, which suggests that clearance was maintained throughout 12 months. In contrast, the majority of lesions not treated in the original study, both clinical and subclinical (which represent the field of cancerization), were still largely present one year later. However, once these same areas subsequently received treatment in the extension study, they exhibited a clearance rate of 69 percent. The difference between these clearance rates (17% vs 69%) was statistically significant. For overall clearance rates, the difference between those rates in the original study and the extension study (79% vs 69%) was not statistically significant, thereby suggesting that we achieved similar levels of efficacy in both studies.
In a similar study conducted by Lebwohl et al that assessed the 12-month recurrence rates following treatment of AK on the face or scalp with ingenol mebutate gel 0.015%,14 there was a sustained clearance rate of 46 percent at one year after lesions had been treated and an overall reduction in the number of lesions. Another long-term follow-up study of AKs after treatment was conducted by Garbe et al, who enrolled 450 patients with 4–8 clinically visible AKs on the face or scalp.15 These patients were also treated with ingenol mebutate gel 0.015%, and they received follow-up at 26 and 44 weeks. After the initial treatment, 277 patients (61.6%) were completely clear at Week 8,141 had persistent AKs at Week 8, and 62 had emergent AKs at Week 26 or Week 44. Those patients with emergent AKs were then randomized and treated with ingenol mebutate gel or vehicle. Complete clearance rates were significantly higher with ingenol mebutate than with vehicle (59.5% vs 25.0%; P=0.01), demonstrating a long-term benefit of ingenol mebutate for initial and follow-up treatment of AKs.
An interesting finding in both our original study and the extension study involved treatment-resistant lesions, as they provided insight into characteristics of AKs that might render some lesions more refractory to treatment. Of the three treatment-resistant lesions present at the end of the original study, two were also present on Day 0 of the extension study. On noninvasive imaging, both lesions contained elements of seborrheic keratosis, benign lesions that feature areas of thickened and well-defined architectural plateaus on OCT (Figure 5, yellow arrows). Reports in the literature describe seborrheic keratosis displaying a classic appearance on OCT of a markedly thickened epithelium, which might contain hypodense structures corresponding to horn pseudocysts on histopathology.16 More importantly, researchers have observed a reduction in treatment response to ingenol mebutate 0.05% gel among seborrheic keratotic lesions.17 The advantage of using OCT to diagnose such AKs that contain mixed morphology is the ability to detect subtle architectural changes that would otherwise be indistinguishable with the naked eye and even with dermoscopy. Recognition of these atypical AKs might facilitate more optimal therapy that is tailored to the patient, improve treatment response, and reduce the risk of recurrence.
One limitation of the extension study was that almost half of the patients in the original study could not be enrolled. These patients were excluded because they had received another treatment for AK during the intervening year, were unavailable, or were otherwise unable to participate. The relatively small cohort size was another limitation, as was the homogeneity of the patients with respect to race and gender. Furthermore, the study was performed at a single center. Finally, although it was assumed for purposes of the extension phase that the untreated lesions at Day 60 of the original study were the same ones that persisted for one year, the possibility that these lesions cleared spontaneously in the interim and were replaced with recurrent or new lesions cannot be excluded.
CONCLUSION
OCT has been shown to be an effective diagnostic tool for the clinician for detecting the presence of AKs, as well as early actinic damage due to the chronic effects of field cancerization. OCT as a noninvasive imaging technology permits this diagnosis while avoiding a biopsy, based on the high diagnostic correlation between OCT and histopathology that we demonstrated in our original study. A significant number of clinical and subclinical lesions that cleared in the original study following treatment with ingenol mebutate gel 0.015% remained clear one year later, while most of the untreated lesions, both clinical and subclinical, were still present. The majority of these formerly untreated lesions did clear, however, after appropriate treatment with ingenol mebutate gel 0.015% in the extension study. These results suggest that ingenol mebutate 0.015% remains an effective agent as a field therapy for both AK and subclinical actinic damage, and that it can provide reliable, long-term clearance. OCT can be particularly helpful to clinicians for detecting early precancerous lesions and distinguishing AKs that contain specific morphologic elements that render them difficult to treat. This allows clinicians to fully tailor the ideal therapy for their patients as a component of comprehensive prevention of skin cancer.
REFERENCES
- 1.Schmitt JV, Miot HA. Actinic keratosis: a clinical and epidemiological revision. An Bros Dermatol. 2012;87(3):425–434. doi: 10.1590/s0365-05962012000300012. [DOI] [PubMed] [Google Scholar]
- 2.Feldman SR, Fleischer AB., Jr Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87(4):201–207. [PubMed] [Google Scholar]
- 3.Vatve M, Ortonne JP, Birch-Machin MA, Gupta G. Management of field change in actinic keratosis. Br J Dermotol. 2007;157(Suppl 2):21–24. doi: 10.1111/j.1365-2133.2007.08268.x. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz O, Schwartz M, Feldman E, et al. Defining field cancerization of the skin using noninvasive optical coherence tomography imaging to detect and monitor actinic keratosis in ingenol mebutate 0.015%-treated patients. J Clin Aesthet Dermatol. 2016;9(5):18–25. [PMC free article] [PubMed] [Google Scholar]
- 5.Jorizzo JL, Markowitz O, Lebwohl MG, et al. A randomized, double-blinded, placebo-controlled, multicenter, efficacy and safety study of 3.75% imiquimod cream following cryosurgery for the treatment of actinic keratosis. J Drugs Dermatol. 2010;9(9):1101–1108. [PubMed] [Google Scholar]
- 6.Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? Part 1 : overview and investigational topical agents. Cutis. 2012;89(5):241–250. [PubMed] [Google Scholar]
- 7.Filosa A, Filosa G. Actinic keratosis and squamous cell carcinoma: clinical and pathological features. G Ital Dermatol Venereol. 2015;150(4):379–384. [PubMed] [Google Scholar]
- 8.Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25(11):855–856. doi: 10.1111/exd.13103. [DOI] [PubMed] [Google Scholar]
- 9.Scope A, Marchetti MA. An evolving approach to the detection of melanoma and other skin cancers using in vivo reflectance confocal microscopy. JAMA Dermatol. 2016;152(10):1085–1087. doi: 10.1001/jamadermatol.2016.1966. [DOI] [PubMed] [Google Scholar]
- 10.Gambichler T, Pljakic A, Schmitz L. Recent advances in clinical application of optical coherence tomography of human skin. Clin Cosmet Invest Dermatol. 2015;8:345–354. doi: 10.2147/CCID.S69119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamalis A, Ho D, Jagdeo J. Optical coherence tomography imaging of normal, chronologically aged, photoaged and photodamaged skin: a systematic review. Dermatol Surg. 2015;41(9):993–1005. doi: 10.1097/DSS.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz L, Reinhold U, Bierhoff E, Dirschka T. Optical coherence tomography: its role in daily dermatology practice. JDDG. 2013;11(6):499–507. doi: 10.1111/ddg.12073. [DOI] [PubMed] [Google Scholar]
- 13.Ondo AL, Padilla RS, Miedler JD, et al. Treatment-refractory actinic keratoses successfully treated using simultaneous combination topical 5-fluorouracil cream and imiquimod cream: a case-control study. Dermatol Surg. 2012;38(9):1469–1476. doi: 10.1111/j.1524-4725.2012.02480.x. [DOI] [PubMed] [Google Scholar]
- 14.Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149(6):666–670. doi: 10.1001/jamadermatol.2013.2766. [DOI] [PubMed] [Google Scholar]
- 15.Garbe C, Bassett-Seguin N, Poulin Y, et al. Efficacy and safety of follow-up field treatment of actinic keratosis with ingenol mebutate 0.015% gel: a randomized, controlled 12-month study. Br J Dermatol. 2016;174(3):481–482. doi: 10.1111/bjd.14222. [DOI] [PubMed] [Google Scholar]
- 16.Forsea AM, Carstea EM, Ghervase L, et al. Clinical application of optical coherence tomography for the imaging of non-melanocytic cutaneous tumors: a pilot multi-modal study. J Med Life. 2010;3(4):381–389. [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen R, Freeman M, Zibert JR, et al. Ingenol mebutate 0.05% gel reduces cancer cells in squamous cell carcinoma in situ and shows marginal effect in seborrheic keratosis. JDDG. 2013;11(Suppl 7) Abstract FC-003. [Google Scholar]