Abstract
The northern root-knot nematode, Meloidogyne hapla, is one of the most important nematode pathogens occurring in cold regions. It is a sedentary, biotrophic parasites of plants and overwinter in the soil or in diseased roots. This study showed that the cold tolerance for the second-stage juveniles (J2) of M. hapla was moderate with the 50% survival temperature (S50) of -2.22°C and the fatal temperature was -6°C when cooling at 0.5°C min-1. Cryoprotective dehydration significantly enhance cold tolerance of M. hapla J2 with the lowest S50 of -3.28°C after held being at -1°C for 6 h. Moreover, cold shock and cold acclimation had significant effects on the freezing survival of M. hapla J2. The lethal temperature of eggs was -18°C. Therefore, the cold tolerance of M. hapla is sufficiently favorable to withstand winters in cold temperature environments.
Introduction
The northern root-knot nematode, Meloidogyne hapla, are sedentary, biotrophic parasites of plants with wide host ranges [1]. The second-stage juvenile (J2) of M. hapla infect plant roots and induce root-knots, which affect water and nutrient absorption and translocation of root systems. Infection results in reduced crop yield or quality [2], and consequently causes severe economic losses [3, 4]. M. hapla occurs in cold regions of crop production [4, 5]. According to a previous report, M. hapla is mainly distributed in cool areas, where the mean temperature is -15°C in the coldest month and approximately 27°C in the warmest month or in high altitude mountainous areas [5]. The only infective stage of M. hapla is the J2, which must overcome adversely low-temperature environmental conditions before reaching plant roots. It was shown that M. hapla J2 survived at freezing temperatures [6, 7], and the minimal temperature for development was 8.8°C [8]. Meanwhile, the egg masses also played a key role in overwintering in the soil. M. hapla eggs survived sub-zero temperatures in the field [9, 10] and developed at the low temperature of 6.74°C [11]. This indicates the ability of M. hapla to survive at low temperatures.
Cold tolerance is the ability of nematodes to survive low temperatures in their living environment [12]. There are three main cold tolerance strategies in nematodes [13]. Freezing avoidance is where a nematode body fluid remains a liquid below 0°C to avoid freezing. Freezing tolerance enables nematode survival when their bodies undergo ice formation while showing supercooling ability. Cryoprotective dehydration protects nematodes from low temperatures by dehydration caused by surrounding ice. Cold tolerance has been studied in other nematodes, including entomopathogenic nematodes, Antarctic nematodes, and stem nematodes [14–18]. Steinernema feltiae and Heterorhabditis bacteriophora survived the low temperature of -13°C by cryoprotective dehydration, and the S50 of S. feltiae was -3.73°C [19]. Panagrellus redivivus survived at low temperature by freezing tolerance and cryoprotective dehydration [17]. Marshallagia marshalli survived rapid exposure to temperature below -30°C [20]. One study determined the cold tolerance of six nematodes with cold acclimation (Ditylenchus dipsaci, P. redivivus, Steinernema carpocapase, Panagrolaimus rigidus, Rhabditophanes sp. and Panagrolaimus davidi), while the S50 of P. davidi was -43.6°C lower than the others [12].
Cold acclimation is an adaptive response of organisms to low temperature that increases their capacity to tolerate freezing, and this response has been observed in P. redivivus, P. davidi [21, 22], S. feltiae, S. riobrave, S. carpocapsae, S. anomaly and H. bacteriophora [18, 23]. In a variety of prokaryotes and eukaryotes, cold shock improved cold tolerance by inducing cold shock proteins [24]. The CspA was a major cold-shock protein induced by Escherichia coli when E. coli was subjected to cold shock [25, 26]. Nematodes H. bacteriophora and Trichinella nativa produced proteins to respond to cold shock [27, 28]. However, the effects of cold shock and cold acclimation on the M. hapla J2 are unknown, although they can survive at low temperatures.
In this study, we investigated the effect of low temperature on the survival of M. hapla J2 and the in vitro hatch rate of egg masses. We determined the influence of cryoprotective dehydration, cold acclimation and cold shock, on M. hapla cold tolerance.
Methods and materials
Nematode culture
The M. hapla were generous gift by Congli Wang (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences), and maintained on a nematode-susceptible tomato (L-402) in the greenhouse according to the described by Forge and MacGuidwin[6]. The eggs were collected by root bleaching and centrifugation with 36% (wt/vol) sucrose [29, 30]. Eggs were hatched in sterile distilled water at 25°C under dark conditions. J2 were collected 24 h after hatching and used for the experiment.
Freezing regime
A 50-μl suspension containing approximately 20 M. hapla J2 nematodes were transferred to a 0.5-ml Eppendorf tube and placed in a cooling block. The temperature of cooling block was controlled by a programmable cooling device (Temperature chamber: TEMI990). The samples were cooled from 1°C to various minimum temperatures (Tmin: -2, -3, -4, -5, -6°C) at 0.5°C min-1 and frozen by adding ice crystals (made by AWT) at Tmin and held for 30 min, then rewarmed to 1°C at 0.5°C min-1. The samples were then removed from the cooling block. After thawing, 300 μl AWT was added to the samples, and placed at room temperature for 24 h. Survival was determined by counting the proportion of moving nematodes after a mechanical stimulus by touching nematodes with a homemade eyelash-needle. Control samples were unfrozen at -1°C. Two runs of this regime were used with 5 replicates per run. The temperature at which 50% of the J2 were killed (S50) was determined using a Probit analysis [12, 17, 19].
Cryoprotective dehydration regime
To test whether the cold tolerant mechanism of M. hapla J2 was a cryoprotective dehydration strategy, samples were cooled from 1°C to -1°C at 0.5°C min-1, frozen by inoculating with ice crystals at -1°C and held for a specific period time (2, 6, 12 h) before cooling to Tmin (-3, -4, -5°C) at 0.5°C min-1. They were kept at Tmin for 30 min and finally rewarmed to 1°C at 0.5°C min-1. Two runs of this regime were used with 5 replicates per run. Survival was determined as previously described.
Cold shock
To test the effect of cold shock on the survival of M. hapla J2, samples were cooled from 1°C to -1°C at 0.5°C min-1 and held for 1 h at -1°C. They were rewarmed to 1°C at 0.5°C min-1 in cool block, and then samples were taken from cool block and maintained at room temperature for 1 h [17] before exposed to Tmin (-3, -4, -5°C) using similar methods as in the ‘freezing regime’. Survival was detected as before.
Cold acclimation
To test the effect of cold acclimation on survival of M. hapla J2, samples were acclimated at 4°C for 12 h before cooling to Tmin (-3, -4, -5°C) at 0.5°C min-1, the cold exposure was using the ‘freezing regime’. Survival was detected as before.
Effect of low-temperature on egg mass hatching rates
To test the hatch rate of the egg mass of M. hapla, which was exposed to low temperature, seven temperature treatments (Tmin: -2, -6, -10, -14, -15, -16, and -18°C) were used. Similar sizes of fresh egg masses were chosen and sterilized by 0.4% NaOCl solution, then placed in the homemade hatching pond. Samples cooled from 1°C to Tmin at 0.5°C min-1, kept at Tmin for 30 min and then warmed to 1°C at 0.5°C min-1, finally the samples were removed from the cooling block. Control samples were kept at 25°C. All treatments were hatched in sterile distilled water at 25°C. Each treatment had 3 replicates per run, and two runs of this regime were used. The number of eggs in each egg-mass were 672 ± 5.5 (mean ± SE) on average. After 10 days, the number of hatching nematodes compared to the proportion of eggs were examined according to the formula as below:
Statistical analysis
All statistical analyses were calculated by using SPSS v. 17.0 [31]. Probit analysis models were used to determine the temperature at which 50% of nematodes were killed (S50). The minimum temperatures (Tmin) were log10 transformed to linearize the data. The relative median potency (RMP) estimated the difference of the S50 between two groups. Significant differences were defined between groups, if the 95% confidence limits (CL) of RMP estimation does not encompass the value 1. The effect of treatments on survival were tested using a factorial ANOVA [12, 17, 19].
Results
Freezing regime and cryoprotective dehydration
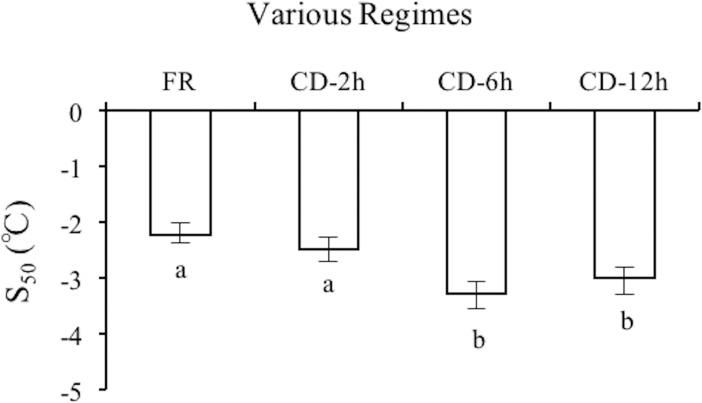
The effect of temperature on survival of M. hapla J2 in the freezing regime is significant. With the temperature reduced, survival decreased significantly (Welch test, Alpha = 0.05, df = 3; P < 0.001) (S1 Fig). The S50 values of freezing regime was -2.22°C (95%CL = -2.01, -2.38°C). Moreover, the S50 values were significantly decreased with an increased freezing time at -1°C after 2–6 h, which was lower 1.06°C after 6 h compared to the freezing regime (RMP = 1.47; 95%CL = 1.23, 2.10), but there was no significant difference between 6 h and 12 h (Fig 1).
Fig 1. The S50 values of M. hapla J2 exposed to freezing regime and cryoprotective dehydration.
FR = freezing regime, CD-2 h = Cryoprotective dehydration regime that held for 2 h at -1°C before cooled to Tmin, CD-6 h = Cryoprotective dehydration regime that held for 6 h at -1°C before cooled to Tmin, CD-12 h = Cryoprotective dehydration regime that held for 12 h at -1°C before cooled to Tmin. The bars are the estimations in the 95% confidence limits. The different lowercase letters on the bars represent significantly different among various regimes, according to RMP estimates. N = 10.
Cold shock and cold acclimation
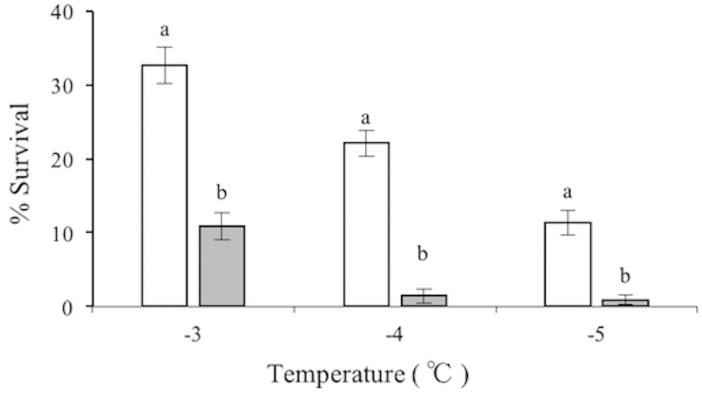
Survival of M. hapla J2 subjected to cold shock at -1°C for 1 h was significantly increased (S2 Fig). The S50 was -2.58°C (95%CL = -2.34, -2.95°C), lower than the S50 in the freezing regime (RMP = 1.16; 95%CL = 1.05, 1.32). Survival of M. hapla J2 significantly improved by acclimated at 4°C compared to the freezing regime (Fig 2). And that the S50 was -2.79°C (95%CL = -2.58, -3.08°C), which was significantly different from the freezing regime (RMP = 1.26; 95%CL = 1,12, 1,52). Moreover, the S50 values of various regimes were compared in Fig 3. The S50 of cryoprotective dehydration for 6 h was lower than those achieved through cold shock (RMP = 1.27; 95%CL = 1,07, 1,75) and acclimation (RMP = 1.17; 95%CL = 1,04, 1,46), but there were no significant differences between cold shock and acclimation.
Fig 2. The effect of cold acclimation on survival of M. hapla J2.
Samples acclimated at 4°C (open bars), and survival at freezing regime without acclimation (filled bars). The different lowercase letters on the bars represent significantly different (P < 0.05) between treatments at the same temperature. The bars are the mean ± SE in this figure. N = 10.
Fig 3. The S50 values of M. hapla J2 subjected to various regimes.
FR = freezing regime; CD-6 h = Cryoprotective dehydration regime that held for 6 h at -1°C before cooled to Tmin, CS = cold shock at -1°C for 1 h and then kept at room temperature for 1 h before cooled to Tmin, A4 = acclimated at 4°C for 12 h before cooled to Tmin. The bars are the estimations in the 95% confidence limits. The different lowercase letters on the bars represent significantly different among various regimes, according to RMP estimates. N = 10.
Effect of low-temperature on egg mass hatching percentage
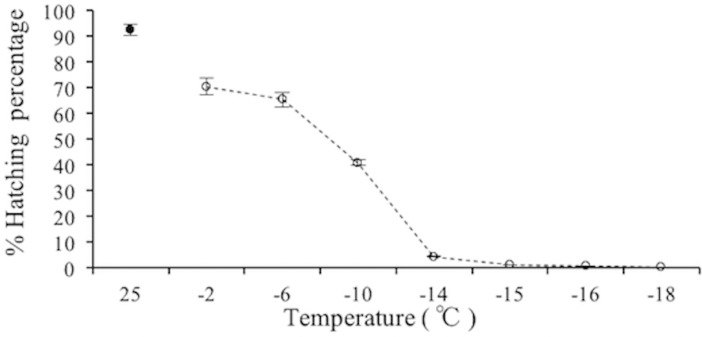
The hatching percentage for egg mass of M. hapla declined with decreasing temperatures (Welch test, Alpha = 0.05, df = 6; P < 0.001) (Fig 4).
Fig 4. The hatching percentage for egg mass of M. hapla at various low temperature.
Open circles = hatching percentage at various low temperature during 10 days, Closed circle = 25°C. The values are the mean ± SE in this figure. N = 6.
Discussion
M. hapla J2 indeed survived at low temperature. Previous studies showed that the M. hapla J2 were frozen spontaneously at -8°C at the freezing rate of 1°C min-1 [7]. However, in our experiment, the freezing rate was 0.5°C min-1 because the physical damage of ice formation decreased using a slow freezing rate [32]. The results indicated the cold tolerance of M. hapla J2 was modest, with the S50 of -2.22°C and fatal temperature of -6°C in the freezing regime. Comparatively, the S50 of P. davidi was -43.6°C [12].
Inoculating ice could make the surrounding medium freeze rapidly at high subzero temperatures and provide the basic environment of cryoprotective dehydration of nematodes [33]. Cryoprotective dehydration [13, 33–36] had a significant effect on cold tolerance of some nematodes, such as P. davidi, S. feltiae, and H. bacteriophora [16, 19]. In our freezing regime, the S50 of M. hapla J2 was -2.22°C, and the value significantly decreased with increased time at -1°C in the cryoprotective dehydration regime. This result might explain that cryoprotective dehydration had an effect on the cold tolerance of M. hapla J2. Moreover, a previous study showed that following exposure to -15°C for 10 min, the cuticle of M. incognita J2 had been torn away from the body by freezing but not in the M. hapla J2 [7]. Thus, the cold tolerance of this species may have been aided by cryoprotective dehydration and freezing tolerance.
Survival of M. hapla J2 was improved significantly by cold shock at -1°C for 1 h, and the S50 was -2.58°C, which was lower than the freezing regime. Cold shock occurs in a variety of organisms [24]. H. bacteriophora induces the trehalose-6-phosphate synthase by cold shock [27], and Hsp70 was markedly increased to in response to cold shock in Trichinella native [28], while, cold shock had no significant effect on survival of P. redivivus [17] and S. feltiae [19].
M. hapla J2 that were acclimated at 4°C for 12 h showed a significant enhancement in survival. These were similar results to those found by Forge and MacGuidwin [6]. Cold acclimation response has been studied on a variety of nematode species [33]. The supercooling points of P. redivivus were decreased by cold acclimation, enabling survival at lower temperatures [21]. The freezing tolerance of S. feltiae, S. anomaly and H. bacteriophora was increased after cold acclimation [18]. Moreover, cold acclimation response induced trehalose accumulation in entomopathogenic nematodes (S. feltiae, S. riobrave, S. carpocapsae) [23] and P. davidi [22]. Meanwhile, calcium or calmodulin-mediated signaling played a pivotal role in response to cold acclimation in plants [37]. However, the mechanism of cold acclimation and cold shock effects on the survival of M. hapla J2 needs to be further investigated.
In nature, nematodes usually overwinter by egg masses in the plant debris or soil when the soil temperature is subzero. In this study, the percentage of egg-hatching for M. hapla within 10 days was 65.30% at -6°C and 4.27% at -14°C, indicating that M. hapla survived at low temperatures, which was similar to the results by Daulton et al. [9]. However, pre-exposure to 4, 12 and 18°C for two weeks did not significantly affect the hatch rate of the M. hapla egg masses [7].
We found that the lethal temperature of M. hapla J2 was -6°C with freezing by adding ice, and cryoprotective dehydration improved the cold tolerance of M. hapla J2. The lethal temperature of M. hapla egg mass in our experiments was -18°C after freezing spontaneously. Moreover, cold acclimation and cold shock significantly improved the cold tolerance of M. hapla J2, which is advantageous for withstanding the winter in cold environments.
Supporting information
Treatments were frozen by adding ice (open circle), and unfrozen (closed circle). The values are the mean ± SE in this figure. N = 10.
(TIFF)
Samples cold shocked at -1°C for 1 h and then kept at room temperature for 1 h before being cooled to Tmin (filled circles), and survival at freezing regime at the corresponding test temperature (-3, -4, -5°C) without cold shock (open circles). The values are the mean ± SE in this figure. N = 10.
(TIFF)
Acknowledgments
We thank Dr. Congli Wang, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, for providing some root knot nematode material for this study and for helpful comments on the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant numbers: 31471748), Mount Tianzhu Scholar Funding Scheme, Special Fund for Agro-scientific Research in the Public Interest (grant numbers: 201103018).(LJC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Perry RN, Moens M, Starr JL. Root-knot nematodes: CABI; 2009:1–17. [Google Scholar]
- 2.Gheysen G, Mitchum MG. How nematodes manipulate plant development pathways for infection. Current Opinion in Plant Biology. 2011;14(4):415–21. doi: 10.1016/j.pbi.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 3.Koenning S, Overstreet C, Noling J, Donald P, Becker J, Fortnum B. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. Journal of nematology. 1999;31(4S):587–618. [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor A, Sasser J. Biology, identification and control of root-knot nematodes North Carolina State University Graphics; 1978:35–42. [Google Scholar]
- 5.Taylor AL, Sasser JN, Nelson L . Relationship of climate and soil characteristics to geographical distribution of Meloidogyne species in agricultural soils: North Carolina State University/AID; 1982. 1–8 p. [Google Scholar]
- 6.Forge T, MacGuidwin A. Cold hardening of Meloidogyne hapla second-stage juveniles. Journal of nematology. 1990;22(1):101 [PMC free article] [PubMed] [Google Scholar]
- 7.Sayre R. Cold-Hardiness of Nematodes. I. Effects of Rapid Freezing On the Eggs and Larvae of Meloidogyne Incognita and M. Hapla 1). Nematologica. 1964;10(1):168–79. [Google Scholar]
- 8.Vrain T, Barker K, Holtzman G. Influence of low temperature on rate of development of Meloidogyne incognita and M. hapla larvae. Journal of Nematology. 1978;10(2):166–70. [PMC free article] [PubMed] [Google Scholar]
- 9.Daulton RA, Nusbaum C. The Effect of Soil Temperature On the Survival of the Root-Knot Nematodes Meloidogyne Javanica and M. Hapla 1). Nematologica. 1961;6(4):280–94. [Google Scholar]
- 10.Vrain T. Influence of chilling and freezing temperatures on infectivity of Meloidogyne incognita and M. hapla. Journal of nematology. 1978;10(2):177–80. [PMC free article] [PubMed] [Google Scholar]
- 11.Vrain T, Barker K. Influence of low temperature on development of Meloidogyne incognita and M. hapla eggs in egg masses. Journal of nematology. 1978;10(4):311–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Smith T, Wharton D, Marshall C. Cold tolerance of an Antarctic nematode that survives intracellular freezing: comparisons with other nematode species. Journal of Comparative Physiology B. 2008;178(1):93–100. [DOI] [PubMed] [Google Scholar]
- 13.Wharton D. The environmental physiology of Antarctic terrestrial nematodes: a review. Journal of Comparative Physiology B. 2003;173(8):621–8. [DOI] [PubMed] [Google Scholar]
- 14.Brown I, Gaugler R. Survival of steinernematid nematodes exposed to freezing. Journal of thermal biology. 1998;23(2):75–80. [Google Scholar]
- 15.Wharton D, Young S, Barrett J. Cold tolerance in nematodes. Journal of Comparative Physiology B. 1984;154(1):73–7. [Google Scholar]
- 16.Wharton DA, Goodall G, Marshall CJ. Freezing survival and cryoprotective dehydration as cold tolerance mechanisms in the Antarctic nematode Panagrolaimus davidi. Journal of Experimental Biology. 2003;206(2):215–21. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi M, Wharton DA. The oatmeal nematode Panagrellus redivivus survives moderately low temperatures by freezing tolerance and cryoprotective dehydration. Journal of Comparative Physiology B. 2011;181(3):335–42. [DOI] [PubMed] [Google Scholar]
- 18.Brown IM, Gaugler R. Cold tolerance of steinernematid and heterorhabditid nematodes. Journal of Thermal Biology. 1996;21(2):115–21. http://dx.doi.org/10.1016/0306-4565(95)00033-X. [Google Scholar]
- 19.Ali F, Wharton DA. Cold tolerance abilities of two entomopathogenic nematodes, Steinernema feltiae and Heterorhabditis bacteriophora. Cryobiology. 2013;66(1):24–9. doi: 10.1016/j.cryobiol.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Carlsson A, Irvine R, Wilson K, Coulson S. Adaptations to the Arctic: low-temperature development and cold tolerance in the free-living stages of a parasitic nematode from Svalbard. Polar biology. 2013;36(7):997–1005. [Google Scholar]
- 21.Mabbett K, Wharton DA. Cold-tolerance and acclimation in the free-living nematode, Panagrellus redivivus. Revue Nématol. 1986;9(2):167–70. [Google Scholar]
- 22.Wharton D, Judge K, Worland M. Cold acclimation and cryoprotectants in a freeze-tolerant Antarctic nematode, Panagrolaimus davidi. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 2000;170(4):321–7. [DOI] [PubMed] [Google Scholar]
- 23.Jagdale G, Grewal P. Acclimation of entomopathogenic nematodes to novel temperatures: trehalose accumulation and the acquisition of thermotolerance. International journal for parasitology. 2003;33(2):145–52. [DOI] [PubMed] [Google Scholar]
- 24.Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Current opinion in microbiology. 1999;2(2):175–80. doi: 10.1016/S1369-5274(99)80031-9 [DOI] [PubMed] [Google Scholar]
- 25.Jones PG, VanBogelen RA, Neidhardt FC. Induction of proteins in response to low temperature in Escherichia coli. Journal of Bacteriology. 1987;169(5):2092–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein J, Pollitt NS, Inouye M. Major cold shock protein of Escherichia coli. Proceedings of the National Academy of Sciences. 1990;87(1):283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagdale GB, Grewal PS, Salminen SO. Both heat-shock and cold-shock influence trehalose metabolism in an entomopathogenic nematode. Journal of Parasitology. 2005;91(5):988–94. doi: 10.1645/GE-504R.1 [DOI] [PubMed] [Google Scholar]
- 28.Martinez J, Perez-Serrano J, Bernadina W, Rodriguez-Caabeiro F. Stress response to cold in Trichinella species. Cryobiology. 2001;43(4):293–302. doi: 10.1006/cryo.2001.2363 [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Lower S, Williamson VM. Application of pluronic gel to the study of root-knot nematode behaviour. Nematology. 2009;11(3):453–64. [Google Scholar]
- 30.Fudali SL, Wang C, Williamson VM. Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Molecular Plant-Microbe Interactions. 2013;26(1):75–86. doi: 10.1094/MPMI-05-12-0107-R [DOI] [PubMed] [Google Scholar]
- 31.Inc S. SPSS regression models 12.0: SPSS Inc.; 2003. [Google Scholar]
- 32.Wharton D, Goodall G, Marshall C. Freezing rate affects the survival of a short-term freezing stress in Panagrolaimus davidi, an Antarctic nematode that survives intracellular freezing. CryoLetters. 2002;23(1):5–10. [PubMed] [Google Scholar]
- 33.Perry RN, Wharton DA. Molecular and physiological basis of nematode survival: CABI; 2011:182–198. [Google Scholar]
- 34.Holmstrup M, Westh P. Dehydration of earthworm cocoons exposed to cold: a novel cold hardiness mechanism. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 1994;164(4):312–5. [Google Scholar]
- 35.Holmstrup M, Bayley M, Ramløv H. Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates. Proceedings of the National Academy of Sciences. 2002;99(8):5716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wharton D, Downes M, Goodall G, Marshall C. Freezing and cryoprotective dehydration in an Antarctic nematode (Panagrolaimus davidi) visualised using a freeze substitution technique. Cryobiology. 2005;50(1):21–8. doi: 10.1016/j.cryobiol.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 37.Yang T, Chaudhuri S, Yang L, Du L, Poovaiah B. A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. Journal of Biological Chemistry. 2010;285(10):7119–26. doi: 10.1074/jbc.M109.035659 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatments were frozen by adding ice (open circle), and unfrozen (closed circle). The values are the mean ± SE in this figure. N = 10.
(TIFF)
Samples cold shocked at -1°C for 1 h and then kept at room temperature for 1 h before being cooled to Tmin (filled circles), and survival at freezing regime at the corresponding test temperature (-3, -4, -5°C) without cold shock (open circles). The values are the mean ± SE in this figure. N = 10.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.