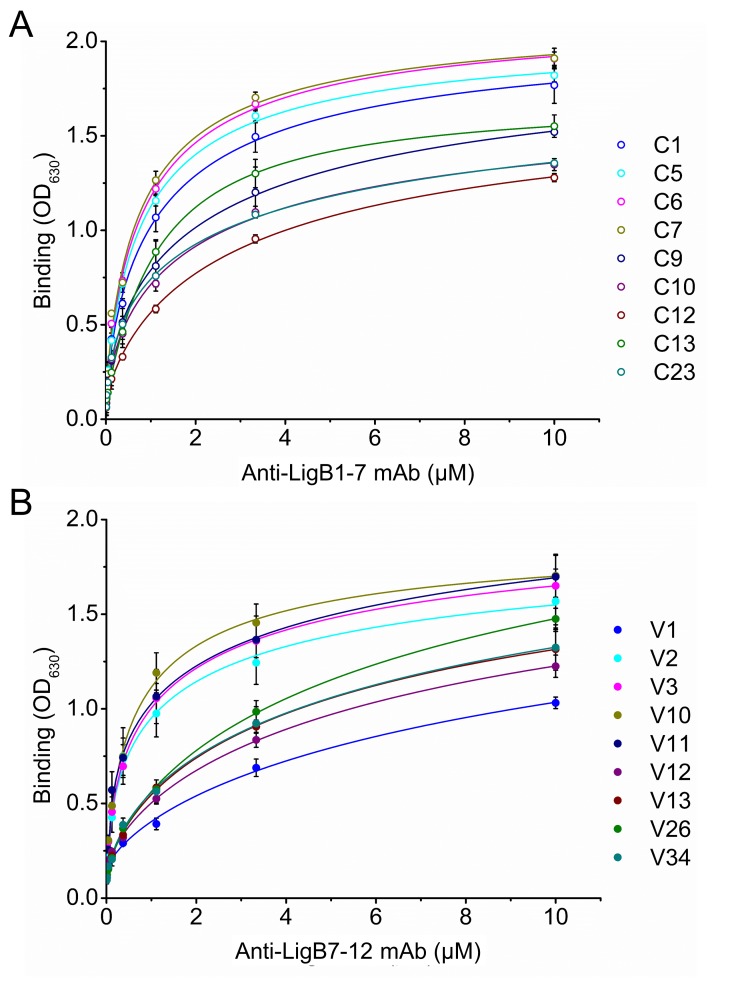
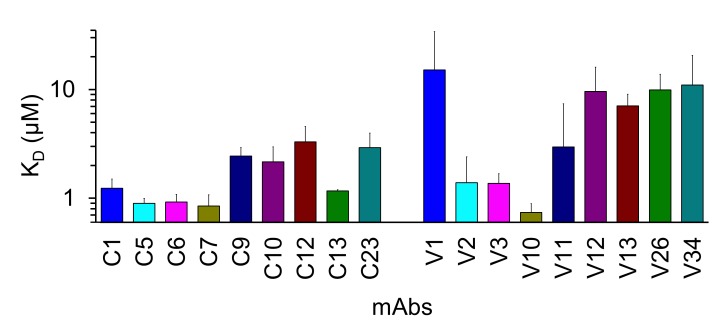
Figure 3. Equilibrium binding for anti-LigB mAbs.
The equilibrium dissociation constants (KD) for mAbs from library C (A) and library V (B) were determined from dose-dependent binding curves. Increasing concentrations of purified anti-LigB mAbs (0.00686, 0.0137, 0.0412, 0.123, 0.370, 1.11, 3.33 and 10 µM) were incubated with LigB antigen (1 μM) immobilized on microtiter plates. The binding interaction was subsequently detected by ELISA using HRP-conjugated anti-mouse antibodies. All experiments were conducted in three trials, the mean ±S.D. of which were shown in bar charts.