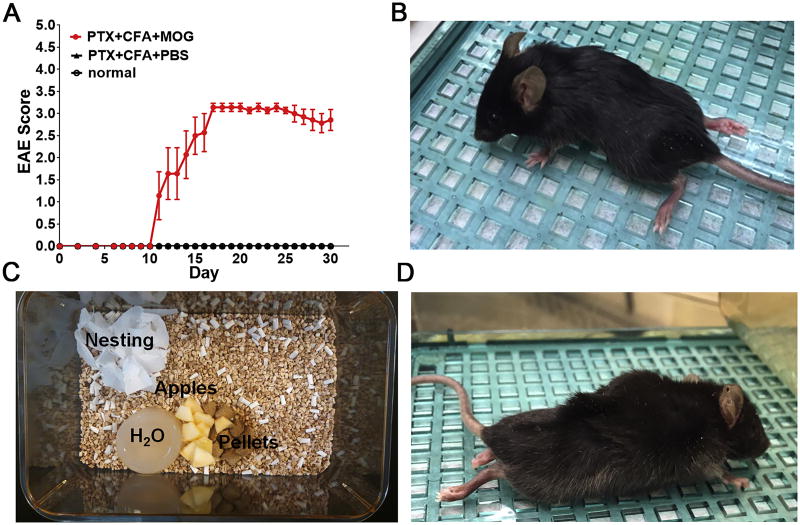
Figure 3. EAE expected outcome.
(A) Eight-week-old female C57BL/6 mice were injected with either PTx+CFA+MOG35–55 (EAE group-red circle) or PTx+CFA+DPBS (no MOG35–55 control group-black triangle), or not injected (normal control group-black circle). EAE scores are shown as mean±SEM; n = 8 mice/group. The EAE group displayed clinical symptoms beginning at post-induction Day ∼10. (B) A symptomatic mouse with a clinical score of 3.5. (C) Typical supplements for EAE mice. Food pellets wetted with water, apple slices, a non-wetting water replacement, and strips of nesting material are commonly added to cages. (D) A dehydrated mouse exhibiting “tenting” following the pinch test.