Abstract
Galectin-3 has been proposed as a novel biomarker of heart failure and cardiac fibrosis, and may also be associated with fibrosis of other organs such as the kidney. To determine this, we prospectively analyzed data from 9,148 Atherosclerosis Risk in Communities (ARIC) Study participants with measured plasma galectin-3 levels (baseline, visit 4, 1996-98) and without prevalent chronic kidney disease (CKD) or heart failure. We identified 1,983 incident CKD cases through December 31, 2013 over a median follow-up of 16 years. At baseline, galectin-3 was cross-sectionally associated with estimated glomerular filtration rate and urine albumin-to-creatinine ratio; both significant. The results were adjusted for age, sex, race-center, education, physical activity, smoking status, body mass index, systolic blood pressure, anti-hypertensive medication use, history of cardiovascular disease, diabetes, fasting blood glucose, and rs4644 (a single nucleotide polymorphism of galactin-3). There was a significant, graded, and positive association between galectin-3 and incident CKD (quartile 4 vs. 1 hazard ratio: 2.22 (95% confidence interval: 1.89, 2.60)). The association was attenuated but remained significant after adjustment for estimated glomerular filtration rate, urine albumin-to-creatinine ratio, troponin T, and N-terminal pro-brain natriuretic peptide (quartile 4 vs. 1 hazard ratio: 1.75 (95% confidence interval: 1.49, 2.06)), and was stronger among those with hypertension at baseline (significant interaction). Thus, in this community-based population, higher plasma galectin-3 levels were associated with an elevated risk of developing incident CKD, particularly among those with hypertension.
Keywords: galectin-3, fibrosis, chronic kidney disease, hypertension
Introduction
Galectin-3 is a 35-kDa, soluble, β-galactoside-binding lectin composed of 250 amino acids which has multiple biological functions.1, 2 A total of 15 galectins have been identified and are classified into three forms: dimeric, tandem, and chimeric.3 When concentrations of galectin-3 are high, this chimeric-type of galectin is found as a multimeric complex allowing for adhesion between cells and the extracellular matrix to facilitate fibrogenesis.2 Recently, galectin-3 has been proposed as a novel biomarker of heart failure due to its involvement in myocardial fibrosis in addition to inflammation, immunity, and adhesion.4-6 Beyond myocardial fibrosis, elevated galectin-3 levels may be associated with the development of fibrosis of other organ tissues, such as the kidney, and may increase the risk of developing chronic kidney disease (CKD) through its other mechanisms of action.7-9
Two epidemiologic studies have been conducted in general population samples (Framingham Heart Study and Cardiovascular Health Study) yielding conflicting results about the role of galectin-3 in CKD.10, 11 These prior studies included approximately 3,000 participants each and consisted primarily of Caucasian individuals. They were able to prospectively identify several hundred incident CKD cases over 3-10 years of follow-up. Given the promising yet inconsistent findings, further research is warranted to better understand the association between galectin-3 and CKD and to provide more broadly generalizable results.
The objective of the present study was to investigate the prospective association between plasma levels of galectin-3 and risk of incident CKD in a community-based population consisting of African-American and Caucasian adults.
Results
Baseline Characteristics
Those with higher plasma levels of galectin-3 were more likely to be older, female, African-American, and to have completed less education (p<0.001 for all comparisons; Table 1). Higher levels of galectin-3 was also associated with worse health status, including higher body mass index and blood levels of NT-proBNP, and a greater burden of risk factors, including elevated blood pressure and diabetes (p<0.001 for all comparisons).
Table 1. Baseline Characteristicsa of Study Participants According to Quartile of Plasma Level of Galectin-3.
| Quartile of Plasma Level of Galectin-3 (Range, ng/mL) | P-valueb | ||||
|---|---|---|---|---|---|
|
| |||||
| Quartile 1 (4.4-12.0) | Quartile 2 (12.1-14.1) | Quartile 3 (14.2-16.7) | Quartile 4 (16.8-91.6) | ||
| N (%) | 2,367 (25.9%) | 2,182 (23.9%) | 2,303 (25.2%) | 2,296 (25.1%) | -- |
| Galectin-3, ng/mL | 10.3 (1.3) | 13.1 (0.6) | 15.4 (0.7) | 20.2 (4.7) | <0.001 |
| Age, years | 61.7 (5.4) | 62.3 (5.6) | 63.1 (5.6) | 64.2 (5.6) | <0.001 |
| Female | 39.5% (936) | 51.3% (1,119) | 62.4% (1,437) | 72.6% (1,668) | <0.001 |
| African-American | 15.3% (363) | 18.4% (402) | 20.7% (477) | 25.6% (588) | <0.001 |
| Education | |||||
| Less than high school | 14.0% (331) | 17.1% (374) | 18.0% (414) | 23.3% (535) | |
| High school | 41.9% (991) | 42.3% (923) | 42.4% (976) | 42.6% (977) | <0.001 |
| College or above | 44.1% (1,045) | 40.6% (885) | 39.6% (913) | 34.1% (784) | |
| Smoking status | |||||
| Current | 14.2% (335) | 15.4% (337) | 13.3% (307) | 14.5% (332) | |
| Former | 47.9% (1,133) | 43.9% (958) | 44.0% (1,013) | 39.5% (908) | <0.001 |
| Never | 38.0% (899) | 40.7% (887) | 42.7% (983) | 46.0% (1,056) | |
| Physical activityc | 2.5 (2.0, 3.3) | 2.5 (2.0, 3.3) | 2.5 (2.0, 3.0) | 2.3 (1.8, 3.0) | <0.001 |
| Body mass index | 28.0 (4.7) | 28.2 (5.2) | 28.9 (5.5) | 30.0 (6.5) | <0.001 |
| Systolic blood pressure, mmHg | 125.3 (17.5) | 126.5 (18.6) | 127.6 (18.8) | 129.5 (19.8) | <0.001 |
| Anti-hypertensive medication use | 42.9% (1,015) | 47.3% (1,031) | 51.2% (1,178) | 65.6% (1,506) | <0.001 |
| History of CVD | 8.0% (190) | 8.6% (188) | 10.3% (237) | 11.9% (273) | <0.001 |
| Diabetes status | 17.9% (423) | 16.7% (364) | 19.5% (449) | 23.9% (549) | <0.001 |
| Fasting blood glucose, mg/dL | 109.1 (33.1) | 108.4 (32.9) | 110.7 (39.2) | 111.8 (39.2) | 0.85 |
| Troponin Tc, ng/L | 5.0 (1.5, 7.0) | 5.0 (1.5, 8.0) | 4.0 (1.5, 8.0) | 5.0 (1.5, 9.0) | <0.001 |
| NT-proBNPc, pg/mL | 55.2 (26.9, 108.6) | 64.6 (30.4, 124.0) | 66.9 (34.3, 128.6) | 89.4 (44.8, 170.9) | <0.001 |
| eGFR-Crc, mL/min/1.73 m2 | 92.0 (81.9, 98.0) | 90.8 (80.3, 97.3) | 88.8 (77.0, 95.6) | 83.3 (68.4, 93.2) | <0.001 |
| eGFR-Cysc, mL/min/1.73 m2 | 92.7 (81.0, 102.1) | 88.8 (77.4, 98.8) | 84.3 (73.5, 95.9) | 73.6 (62.0, 87.3) | <0.001 |
| UACR, mg/g | |||||
| <30 | 95.1% (2,251) | 94.4% (2,060) | 93.1% (2,143) | 87.3% (2,005) | <0.001 |
| 30-300 | 4.6% (109) | 4.7% (103) | 5.9% (137) | 9.1% (210) | |
| >300 | 0.3% (7) | 0.9% (19) | 1.0% (23) | 3.5% (81) | |
| rs4644 | |||||
| AA | 40.1% (949) | 12.3% (269) | 5.2% (120) | 2.1% (49) | 0.39 |
| AC | 50.1% (1,187) | 59.9% (1,306) | 46.5% (1,071) | 30.2% (694) | |
| CC | 9.8% (231) | 27.8% (607) | 48.3% (1,112) | 67.6% (1,553) | |
% (n) for categorical variables; mean (standard deviation) for continuous variables unless otherwise stated
Cochran-Armitage trend tests for categorical variables and linear regression for continuous variables were used to test for trend in baseline characteristics across quartiles of plasma level of galectin-3.
Median (25th percentile, 75th percentile)
CVD, cardiovascular disease; eGFR-Cr, estimated glomerular filtration rate based on creatinine; eGFR-Cys, estimated glomerular filtration rate based on cystatin C; NT-proBNP, N-terminal pro-brain natriuretic peptide; UACR, urine albumin-to-creatinine ratio
Cross-Sectional Analysis of Kidney Measures and Galectin-3
The median (25th, 75th percentiles) plasma level of galectin-3 was 14.2 (12.0, 16.8) ng/mL. The distribution of plasma galectin-3 levels was right-skewed with values ranging from 4.4 up to 91.6 ng/mL (Supplemental Figure 1). In this community-based population, participants had well-preserved kidney function at baseline with median (25th, 75th percentiles) eGFR of 89.4 (77.2, 96.2) and 7.5% of participants had moderately or severely increased albuminuria at baseline (UACR >30 mg/g).
At baseline, galectin-3 was inversely associated with eGFR (r = -0.23, p<0.001; Supplemental Figure 2) and directly associated with urine albumin-to-creatinine ratio (UACR) (r = 0.08, p<0.001; Supplemental Figure 3). The correlation between galectin-3 and kidney measures was stronger among those with impaired kidney filtration (eGFR <60 mL/min/1.73 m2: r = -0.42, p<0.001 vs. eGFR ≥60 mL/min/1.73 m2: r = -0.16, p<0.001; 95% CI for the correlation difference: -0.33, -0.18) and kidney damage (UACR >300 mg/g: r = 0.24, p=0.005 vs. UACR ≤300 mg/g: r = 0.06, p<0.001; 95% CI for the correlation difference: -0.34, -0.01).
Prospective Analysis of Galectin-3 and Incident CKD
Over a median follow-up of 16 years, a total of 1,983 participants (22%) developed incident CKD. After adjusting for age, sex, race-center, education level, physical activity, smoking status, body mass index, systolic blood pressure, anti-hypertensive medication use, history of cardiovascular disease, diabetes status, fasting blood glucose, and rs4644, there was a significant, graded, and positive association between plasma levels of galectin-3 and incident CKD (Model 1, HR for quartile 2 vs. 1: 1.25, 95% CI: 1.09, 1.44; HR for quartile 3 vs. 1: 1.53, 95% CI: 1.32, 1.77; HR for quartile 4 vs. 1: 2.22, 95% CI: 1.89, 2.60; p<0.001; Table 2). While the results remained significant, the adjustment for eGFR considerably attenuated the effect estimates (Model 2), whereas very little change in the results was observed after additionally adjusting for UACR (Model 3). Still, the association between galectin-3 and incident CKD remained significant in each model (e.g., Model 3, quartile 4 vs. 1 HR: 1.81, 95% CI: 1.54, 2.13; p<0.001). Similarly, the results were essentially unchanged after accounting for pre-clinical markers of cardiovascular function, i.e. troponin T and NT-proBNP (Model 4).
Table 2. Adjusted Hazard Ratios for Incident Chronic Kidney Disease by Quartile of Plasma Galectin-3.
| Model | Quartile of Plasma Level of Galectin-3 (Range, ng/mL) | P-value | |||
|---|---|---|---|---|---|
|
| |||||
| Quartile 1 (4.4-12.0) | Quartile 2 (12.1-14.1) | Quartile 3 (14.2-16.7) | Quartile 4 (16.8-91.6) | ||
| 1a | 1 [Ref] | 1.25 (1.09-1.44) | 1.53 (1.32-1.77) | 2.22 (1.89-2.60) | <0.001 |
| 2b | 1 [Ref] | 1.17 (1.01-1.34) | 1.35 (1.16-1.56) | 1.84 (1.57-2.17) | <0.001 |
| 3c | 1 [Ref] | 1.17 (1.01-1.34) | 1.34 (1.16-1.55) | 1.81 (1.54-2.13) | <0.001 |
| 4d | 1 [Ref] | 1.15 (1.00-1.32) | 1.31 (1.13-1.51) | 1.75 (1.49-2.06) | <0.001 |
Model 1: adjusted for age, sex, race-center, education level, physical activity, smoking status, body mass index, systolic blood pressure, anti-hypertensive medication use, history of cardiovascular disease, diabetes status, fasting blood glucose, rs4644
Model 2: Model 1 + estimated glomerular filtration rate-creatinine
Model 3: Model 2 + urine albumin-to-creatinine ratio
Model 4: Model 3 + troponin T, N-terminal pro B-type natriuretic peptide
The cubic spline representing the shape of the association between plasma concentrations of galectin-3 and adjusted hazard ratios for the risk of incident CKD demonstrated a nearly linear association at and above plasma galectin-3 levels of 10-15 ng/mL (Figure 1).
Figure 1. Spline of Adjusted* Hazard Ratios (95% CI) for Incident Chronic Kidney Disease According to Baseline Plasma Level of Galectin-3.
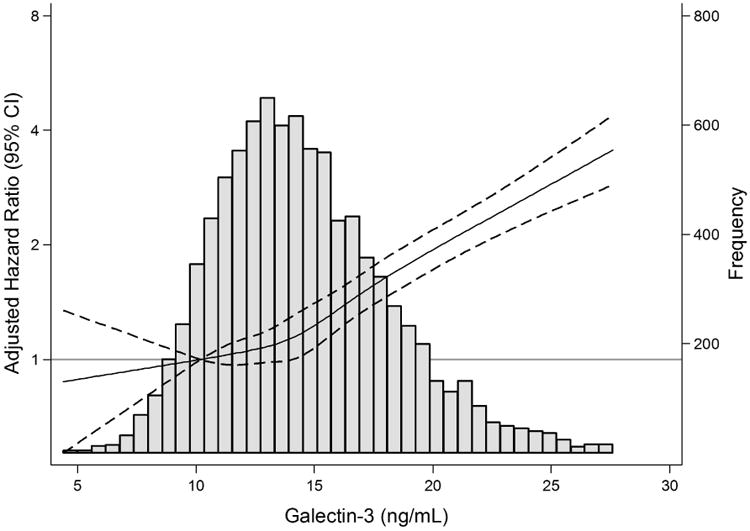
*Adjusted for age, sex, race-center, body mass index, smoking, education, physical activity, systolic blood pressure, anti-hypertensive medication use, history of cardiovascular disease, diabetes, fasting glucose, rs4644, estimated glomerular filtration rate-creatinine, and urine albumin-to-creatinine ratio, troponin T, and N-terminal pro B-type natriuretic peptide
Histogram of baseline plasma level galectin-3 are shown in grey. The solid lines represent the restricted cubic spline with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles for galectin-3. Galectin-3 levels were truncated at the 99th percentile. The 10th percentile of galectin-3 (10.2 ng/mL) was used as the reference point. Dashed lines represent the 95% confidence intervals.
Galectin-3 improved the prediction of incident CKD modestly when added to all models [C statistic (95% CI) for Model 4: 0.713 (0.702, 0.725) vs. Model 4 + galectin-3: 0.718 (0.706, 0.729); p-value for difference in C-statistics=0.002; Table 3].
Table 3. Prediction of Incident Chronic Kidney Disease with Galectin-3 beyond Other Explanatory Factors.
| Model | C-statistic | Difference (95% CI) in C-statistics | P-value for difference in C-statistics |
|---|---|---|---|
| 1a | 0.685 (0.673-0.697) | 1 [Ref] | -- |
| 1a + galectin-3 | 0.695 (0.684-0.707) | 0.011 (0.006-0.015) | <0.001 |
|
| |||
| 2b | 0.706 (0.695-0.718) | 1 [Ref] | -- |
| 2b + galectin-3 | 0.712 (0.700-0.723) | 0.005 (0.002-0.008) | <0.001 |
|
| |||
| 3c | 0.708 (0.697-0.721) | 1 [Ref] | -- |
| 3c + galectin-3 | 0.713 (0.702-0.725) | 0.005 (0.002-0.008) | 0.001 |
|
| |||
| 4d | 0.713 (0.702-0.725) | 1 [Ref] | -- |
| 4d + galectin-3 | 0.718 (0.706-0.729) | 0.004 (0.002-0.007) | 0.002 |
Model 1: adjusted for age, sex, race-center, education level, physical activity, smoking status, body mass index, systolic blood pressure, anti-hypertensive medication use, history of cardiovascular disease, diabetes status, fasting blood glucose, rs4644
Model 2: Model 1 + estimated glomerular filtration rate-creatinine
Model 3: Model 2 + urine albumin-to-creatinine ratio
Model 4: Model 3 + troponin T, N-terminal pro B-type natriuretic peptide
Subgroup Analyses
The association was similar by age (p for interaction = 0.28), sex (p for interaction = 0.37), race (p for interaction = 0.60), and baseline eGFR (p for interaction = 0.23). There was also no significant interaction by diabetes status (p for interaction = 0.30), but the association was qualitatively stronger among those with diabetes (Model 4, HR for quartile 4 vs. 1: 2.28, 95% CI: 1.66, 3.11; p<0.001) compared to those without diabetes (Model 4, HR for quartile 4 vs. 1: 1.55, 95% CI: 1.28, 1.88; p<0.001). The association between galectin-3 and CKD was stronger among those with hypertension (Model 4, HR for quartile 4 vs. 1: 1.94, 95% CI: 1.57, 2.39; p<0.001) than for those without hypertension (Model 4, HR for quartile 4 vs. 1: 1.48, 95% CI: 1.13, 1.95, p=0.003; p for interaction = 0.01; Table 4).
Table 4. Adjusted Hazard Ratios for Incident Chronic Kidney Disease by Quartile of Plasma Galectin-3 Stratified by Hypertension Status.
| Hypertension Status | Model | Quartile of Plasma Level of Galectin-3 (Range, ng/mL) | P-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Quartile 1 (4.4-12.0) | Quartile 2 (12.1-14.1) | Quartile 3 (14.2-16.7) | Quartile 4 (16.8-91.6) | |||
| Hypertension | 1a | 1 [Ref] | 1.43 (1.19-1.72) | 1.73 (1.43-2.10) | 2.57 (2.10-3.14) | <0.001 |
| 2b | 1 [Ref] | 1.33 (1.10-1.60) | 1.51 (1.24-1.83) | 2.11 (1.72-2.60) | <0.001 | |
| 3c | 1 [Ref] | 1.33 (1.10-1.59) | 1.50 (1.24-1.82) | 2.05 (1.67-2.53) | <0.001 | |
| 4d | 1 [Ref] | 1.29 (1.07-1.55) | 1.44 (1.19-1.75) | 1.94 (1.57-2.39) | <0.001 | |
|
| ||||||
| No Hypertension | 1a | 1 [Ref] | 1.02 (0.82-1.27) | 1.28 (1.01-1.61) | 1.79 (1.37-2.34) | <0.001 |
| 2b | 1 [Ref] | 0.96 (0.77-1.20) | 1.16 (0.92-1.46) | 1.55 (1.18-2.02) | 0.001 | |
| 3c | 1 [Ref] | 0.96 (0.77-1.19) | 1.15 (0.91-1.46) | 1.51 (1.15-1.98) | 0.002 | |
| 4d | 1 [Ref] | 0.95 (0.77-1.19) | 1.14 (0.90-1.44) | 1.48 (1.13-1.95) | 0.003 | |
Model 1: adjusted for age, sex, race-center, education level, physical activity, smoking status, body mass index, systolic blood pressure, anti-hypertensive medication use, history of cardiovascular disease, diabetes status, fasting blood glucose, rs4644
Model 2: Model 1 + estimated glomerular filtration rate-creatinine
Model 3: Model 2 + urine albumin-to-creatinine ratio
Model 4: Model 3 + troponin T, N-terminal pro B-type natriuretic peptide
Sensitivity Analyses
When heart failure was modeled as a time-varying covariate, the results remained consistent with the main findings (Supplemental Table 1). For the outcome of rapid eGFR decline, defined as annual eGFR decline of at least 3 mL/min/1.73 m2, the signal persisted for the highest vs. lowest quartile of galectin-3 (Supplemental Table 2). For more advanced kidney disease, a total of 423 (4.6%) participants developed incident kidney failure over a median follow-up of 16 years (Supplemental Table 3). The association between galectin-3 and incident kidney failure persisted in all four models. After account for the competing risk of death prior to the development of CKD, the association between galectin-3 and incident CKD was similar to the main findings (Supplemental Table 4).
Discussion
In this community-based population of 9,148 African-American and Caucasian men and women without pre-existing heart failure, higher plasma galectin-3 levels were associated with an elevated risk of developing new-onset CKD over approximately 16 years of follow-up. These findings persisted even after adjusting for established risk factors, baseline kidney function (eGFR, UACR), and preclinical measures of cardiovascular function (troponin T, NT-proBNP). Galectin-3 improved the prediction of incident CKD beyond established risk factors, kidney measures, and cardiovascular indicators. The magnitude of the association and overall pattern was consistent in several analyses, after accounting for the development of heart failure during follow-up, using alternative outcome definitions including rapid eGFR decline and kidney failure, and incorporating the competing event of death before the development of CKD. Individuals with hypertension appeared to be particularly susceptible to the risk of incident CKD in association with elevated galectin-3 levels.
To the best of our knowledge, only two prior studies have examined the prospective association between galectin-3 and incident CKD. Our findings are consistent with the Framingham Offspring Study (N=2,450), in which higher levels of galectin-3 were associated with incident CKD as well as rapid eGFR decline (defined as ≥3 mL/min/1.73 m2 per year) over 10 years of follow-up even after accounting for age, sex, diabetes, hypertension, proteinuria, and baseline eGFR.10 In contrast, in the Cardiovascular Heart Study (N=2,763), baseline galectin-3 was prospectively associated with incident reduced eGFR (<60 mL/min/1.73 m2) over 7 years of follow-up in unadjusted analyses, but was not statistically significant in adjusted analyses (age, sex, race, baseline eGFR, body mass index, systolic blood pressure, hypertension medications, diabetes, smoking low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, prevalent cardiovascular disease, troponin T, and NT-proBNP) or when using alternative outcomes (i.e. continuous percent eGFR decline; 30% eGFR decline over 3-4 years of follow-up).11 Potential reasons for the discordant findings include older age, greater burden of other risk factors (greater proportion with a history of cardiovascular disease, higher systolic blood pressure level, and higher low-density lipoprotein cholesterol level), lower kidney function, smaller sample size, and shorter follow-up time period in the Cardiovascular Health Study relative to the present study.
We observed that galectin-3 was associated with incident CKD in the overall ARIC study population, and that this association was stronger among those with hypertension at baseline. This finding is supported by previous work demonstrating that galectin-3 is involved in atherosclerotic disease (coronary heart disease), in addition to heart failure.12, 13 Galectin-3 may lead to CKD by promoting atherosclerotic plaque development, and this process may amplified in the setting of existing vascular damage, through its purported role in stimulating the inflammatory cascade, recruiting macrophages, and attracting monocytes.7, 12, 14 In addition to the common underlying atherosclerotic pathway, elevated blood pressure itself may lead to tissue fibrosis in both the kidney and the heart. There is a substantial body of literature showing that aldosterone, which plays a part in the renin-angiotensin system by regulating water retention and thereby influencing blood pressure, can stimulate the secretion of galectin-3 as an intermediate in the fibrotic pathway.14-16 Galectin-3 has been implicated in the development of fibrosis of several organs, including the kidney, through fibroblast proliferation and extracellular matrix remodeling.7, 17 Thus, it may be that elevated levels of galectin-3 indicate a systemic disorder leading to tissue and vascular damage in the heart as well as the kidneys.
An important clinical implication of these study findings is further justification for the use of pharmacotherapy targeting galectin-3 in order to prevent CKD and associated clinical sequelae. Several agents including GCS-100, modified citrus pectin, and N-acetyllactosamine have been investigated in vitro and in animal models to inhibit the activity of galectin-3.18-22 Antagonism of galectin-3 has also demonstrated favorable health outcomes such as reducing renal fibrosis, hypertensive nephropathy, proteinuria, and acute kidney injury.18, 21, 23, 24
In the present study, we observed that galectin-3 levels were moderately related to kidney function measures cross-sectionally, i.e. eGFR and UACR. Prospectively, galectin-3 remained associated with incident CKD, but measures of association were attenuated after adjusting for eGFR and UACR. This finding is consistent with prior research in the Framingham Offspring Study and in a pooled analysis of the 4D Study (Die Deutsche Diabetes Dialyse Studie) (n=1,168 dialysis patients with type 2 diabetes) and the Ludwigshafen Risk and Cardiovascular Health (LURIC) study (n=2,579 patients with coronary angiograms).10, 25 Elevated circulating levels of galectin-3 could theoretically be a consequence of impaired kidney filtration rather than a direct cause of kidney damage. However, in the present study, we were able to establish temporality by conducting a prospective analysis of baseline measurements of galectin-3 and subsequent onset of incident CKD during follow-up. In addition, we demonstrated the persistence of the association between galectin-3 and incident CKD by adjusting for eGFR and UACR in separate regression models.
There are some study limitations and strengths to be considered in the interpretation of our study findings. Plasma levels of galectin-3 were measured at a single time point in the present study. Incorporating repeated measures of galectin-3 levels over time may offer additional information about the risk of subsequent clinical outcomes.26 Genetic variation at rs4644 influences the current galectin-3 assays. Whether isoform-independent assays will yield different results is uncertain. Biochemical measures of kidney function were only available at two time points for the purpose of this study (baseline and follow-up). To address this issue, the visit-based measures were supplemented with information about CKD status during the follow-up period through surveillance efforts, including linkage of the cohort to the USRDS registry and identification of kidney-related hospitalization and deaths. This composite outcome definition has been validated in the ARIC study by comparing to medical chart review and has been used to identify incident CKD in several previous publications.27-31 A major strength of this study, particularly given the conflicting results in the literature, is the consistency of the association across multiple outcome definitions for kidney disease, which lends further credence to the study findings. In light of the present study, we believe galectin-3 is a promising independent risk factor for CKD progression worthy of further exploration across the full spectrum of CKD in future studies. We had limited power to examine effect modification by important clinical characteristics (e.g. diabetes). It may be warranted for future studies to investigate the association between galetin-3 and CKD risk among individuals with diabetes. Another noteworthy strength of the present study is that we employed several strategies to isolate the association between galectin-3 and CKD from the known role of galectin-3 in cardiovascular disease, by restricting the study population to those without heart failure at baseline, adjusting for new-onset heart failure during follow-up as a time-varying covariate, and accounting for markers of preclinical cardiovascular disease (troponin T, NT-proBNP) in the adjusted regression models. Other study strengths relate to the design of the ARIC study including the long-term follow-up, large sample size, representation of African-American and Caucasian men and women, and extensive characterization of study participants for confounder adjustment in multivariable regression models.
In summary, elevated blood levels of galectin-3 were significantly associated with both early and late stages of incident kidney disease in a diverse population with preserved kidney function and without heart failure. Galectin-3 may be useful for identifying individuals with a high risk of developing new-onset CKD and could be a relevant target for pharmacotherapy for the prevention of CKD incidence and progression, especially for individuals with hypertension.
Methods
Study Design
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort of 15,792 men and women, predominantly African-American and Caucasian individuals, who were randomly selected from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington Country, Maryland.32 Participants were middle-aged (45-64 years of age) at the time of study enrollment in 1987-1989 (visit 1). Subsequent follow-up visits occurred in 1990-1992 (visit 2), 1993-1995 (visit 3), 1996-1998 (visit 4), and 2011-2013 (visit 5). For the present study, visit 4 was considered as baseline and participants were followed thereafter for the development of CKD through December 31, 2013. Visit 4 was selected as baseline given the availability of data on galectin-3 and covariates, including kidney measures (eGFR, UACR) and subclinical cardiovascular markers (NT-proBNP, cardiac troponin), and the extensive follow-up period for outcome ascertainment. The ARIC study protocol was approved by the Institutional Review Board at each study site. Participants provided written documentation of informed consent at each study visit.
Study Population
Study participants who attended visit 4 were eligible for the present study (n=11,656). Among this subset, further exclusion criteria were applied: no available measurements of galectin-3 (n=935), prevalent CKD (n=224), prevalent heart failure (n=210), and missing data on covariates (n=1,139). After these exclusions, the analytic study population consisted of 9,148 study participants.
Exposure Assessment
Galectin-3 concentrations were measured in blood specimens collected from participants at visit 4 (1996-1998), processed, and stored as plasma in tubes containing EDTA as an anticoagulant at -70°C until laboratory analysis. Plasma galectin-3 concentrations were measured using a chemiluminescent micro-particle immunoassay on the ARCHITECT i2000SR Immunoassay Analyzer (Abbott Diagnostics, Abbott Park, Illinois).33
Outcome Ascertainment
The main outcome was incident CKD that occurred between baseline (visit 4, 1996-1998) and December 31, 2013. Estimated glomerular filtration rate (eGFR) was calculated using the creatinine-based 2009 CKD Epidemiology (CKD-EPI) Collaboration equation, incorporating serum creatinine measurements at visit 4 and visit 5 (eGFR-Cr).34 Creatinine was measured by the modified kinetic Jaffe method for visit 4 specimens and using the Roche enzymatic method for visit 5 specimens (Roche-Hitachi Modular P chemistry analyzer, Roche Creatininase Plus assay, Hoffman-La Roche, Ltd.). To account for variability in assay methods over time, creatinine values were calibrated to the National Institute of Standards and Technology (NIST) standard and recalibrated using a sample of 200 ARIC study participants.35-37
Incident CKD was defined as meeting any one of the following criteria: 1) eGFR<60 mL/min/1.73 m2 at follow-up (visit 5) accompanied by ≥25% eGFR decline relative to baseline (visit 4) in accordance with recent proposals for quantifying CKD progression; 2) CKD-related hospitalization or death based on ICD-9/10 codes; and 3) end-stage renal disease as identified by the U.S. Renal Data System (USRDS) registry.27, 38 Given that biochemical measures of kidney function (eGFR) were available only at two time points for the purpose of the present study (visit 4 and visit 5), the visit-based measures were supplemented with surveillance-based measures (hospitalizations, deaths, USRDS registry) for the main outcome.
Several alternative kidney disease outcomes were used in order to assess the robustness of our findings. To mirror the analysis conducted in a previously published study, rapid eGFR decline was defined as ≥3 mL/min/1.73 m2 per year.10 We also analyzed more advanced kidney disease, i.e. incident kidney failure, using a composite outcome definition consisting of participants meeting any one of the following criteria: renal replacement therapy (either transplant or dialysis) identified through linkage with the USRDS registry, kidney failure-related hospitalizations and deaths, or eGFR <15 mL/min/1.73 m2 at visit 5.39, 40
Assessment of Other Covariates
A questionnaire was administered by trained interviewers to ascertain information about sociodemographic characteristics, education, medical history, medication use, and health behaviors. During the clinical examination portion of the study visit, body weight and height were assessed and were used for the calculation of body mass index. Two measurements of blood pressure were taken using a random-zero sphygmomanometer and the average of the measurements was used for the analysis. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medication within the preceding two weeks. Fasting blood glucose was measured by the modified hexokinase/glucose-6-phosphate dehydrogenase method. Diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, current use of diabetes medication, or self-reported history of diagnosed diabetes. Incident cases of heart failure were identified using ICD-9/10 codes for heart failure-related hospitalizations and deaths.41
Cardiac troponin T was measured using a high sensitivity sandwich immunoassay (Roche Elecsys T, Roche Diagnostics) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured using an electrochemiluminescent immunoassay, both of which were assessed in plasma specimens on the Cobas e411 autoanalyzer (Roche Diagnostics).
Urine concentrations of creatinine were measured by the modified kinetic Jaffe method, and urine concentrations of albumin were measured by the nephelometric method either on the Dade Behring BN100 or the Beckman Image Nephelometer. The ratio of these measures, i.e. urine albumin-to-creatinine ratio (UACR), was used in the analysis. Cystatin C was measured using a particle-enhanced immunonephelometric assay with a BNII nephelometer at visit 4 (Siemens Healthcare Diagnostics, Erlangen, Germany) and using a particle-enhanced turbidometric assay at visit 5 (Gentian AS, Moss, Norway). Cystatin C values were calibrated and standardized to the International Federation for Clinical Chemists (IFCC) reference, and were used to calculate eGFR based on cystatin C (eGFR-Cys).42-44 Genotyping for the galectin-3 single nucleotide polymorphism (SNP; rs4644), which is the substitution of histidine for proline at position 191, was performed using an exome chip, i.e. the Illumina Infinium HumanExome BeadChip version 1.0 (Illumina, Incorporated, San Diego, California).45
Statistical Analysis
Descriptive statistics (means, proportions, etc.) were used to examine baseline characteristics of the study participants according to quartiles of galectin-3. Quartiles were used to empirically classify participants according to galectin-3 level given the lack of a clinically meaningful cut-point. Differences in these baseline characteristics across quartiles of galectin-3 were tested using Cochran-Armitage trend tests for categorical variables and linear regression for continuous variables. The cross-sectional association between galectin-3 and measures of kidney function (eGFR, UACR) was investigated using scatterplots and Spearman's rank correlation coefficients for the overall study population and within eGFR and UACR subgroups. To quantitatively compare correlation coefficients across eGFR and UACR subgroups, we calculated confidence intervals for the difference between correlation coefficients.46
Cox proportional hazards regression was used to calculate hazard ratios and 95% confidence intervals (CI) for the association between quartiles of galectin-3 and risk of incident CKD during follow-up, incorporating time to the development of CKD and accounting for censoring. Several successive multivariable regression models were run. Model 1 was adjusted for demographic characteristics (age, sex, race-center), socioeconomic status (education level), health behaviors (physical activity, cigarette smoking status), anthropometrics (body mass index), clinical characteristics (systolic blood pressure, anti-hypertensive medication use, hypertension status, fasting blood glucose, diabetes status, history of cardiovascular disease), and the genetic variant for galectin-3 (rs4644). We adjusted for rs4644 since there is evidence that this variant influences binding to the antibody used in the galectin-3 variant. In our study, rs4644 explained 0.4% of the variance in plasma levels of galectin-3. Adjustment for rs4644 removes confounding of the assay by this genetic variant. Secondary analyses without adjustment for rs4644 yielded similar results. Models 2 and 3 were sequentially adjusted for baseline measures of kidney function (eGFR in Model 2 and UACR in Model 3). Model 4 was additionally adjusted for markers of cardiovascular dysfunction (NT-proBNP, troponin T). The assumption of proportionality of hazards was formally tested using Schoenfeld residuals and assessed graphically by examining log-log plots of survival.
Restricted cubic splines with five knots at 5th, 27.5th, 50th, 72.5th, and 95th percentiles were used to visually depict the shape of the association between galectin-3 and CKD risk using Harrell's method.47 Harrell's C-statistic was used to assess model discrimination for the prediction of incident CKD in regression models with and without galectin-3.48
The likelihood ratio test was used to examine interaction by several factors (age, sex, race, eGFR, diabetes, and hypertension) on the association between galectin-3 and incident CKD. Interaction terms were created by multiplying each factor (age, sex, race, eGFR, diabetes, and hypertension) by galectin-3 quartile. The likelihood ratio test compared the fully adjusted model (Model 4) with an interaction term along with the main effects to the fully adjusted model (Model 4) without this interaction term. Stratified analyses were conducted when there was evidence of effect modification.
To assess the robustness of the findings, several sensitivity analyses were conducted. In addition to restricting the analysis to participants without heart failure at baseline and adjusting for preclinical markers of cardiovascular function in Model 4 (troponin T, NT-proBNP), we accounted for the development of heart failure during follow-up using a time-varying covariate. We conducted sensitivity analyses using alternative kidney disease outcome definitions including rapid eGFR decline and kidney failure. We evaluated the galectin-3 and CKD associations after accounting for the competing risk of death prior to the development of CKD using the Fine and Gray method.49
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of Health.
Some of the data reported here have been supplied by the United States Renal Data System registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the U.S. government.
Sources of support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This research was also supported by grants R01 DK089174 and R01 HL134320. Dr. Selvin is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K24 DK106414). Dr. Coresh is also partially supported by the Chronic Kidney Disease Biomarkers Consortium of the NIDDK (U01 DK085689). Dr. Rebholz is supported by a mentored research scientist development grant from the NIDDK (K01 DK107782).
Several authors have received grant support from Abbott (Dr. Ballantyne), Denka Seiken (Dr. Hoogeveen), the National Kidney Foundation (Drs. Coresh and Grams), Kidney Disease: Improving Global Outcomes (KDIGO; Dr. Grams), the Foundation for the National Institutes of Health (Dr. Selvin), and the National Institutes of Health (Drs. Rebholz, Selvin, Ballantyne, Grams, and Coresh). Dr. Ballantyne reports being a paid consultant for Abbott. Dr. Coresh is a co-investigator on a provisional patent (“Precise estimation of glomerular filtration rate from multiple biomarkers”; filed August 15, 2014), and Dr. Hoogeveen is a co-investigator on a provisional patent file by Roche for use of biomarkers in heart failure prediction.
Footnotes
Disclosure: Otherwise, the authors report no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu FT, Hsu DK, Zuberi RI, et al. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 2.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 3.Barondes SH, Cooper DN, Gitt MA, et al. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 4.de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer RA, Voors AA, Muntendam P, et al. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–817. doi: 10.1093/eurjhf/hfp097. [DOI] [PubMed] [Google Scholar]
- 6.McEvoy JW, Chen Y, Halushka MK, et al. Galectin-3 and Risk of Heart Failure and Death in Blacks and Whites. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvier L, Martinez-Martinez E, Miana M, et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. doi: 10.1016/j.jchf.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Iacoviello M, Aspromonte N, Leone M, et al. Galectin-3 Serum Levels Are Independently Associated With Microalbuminuria in Chronic Heart Failure Outpatients. Res Cardiovasc Med. 2016;5:e28952. doi: 10.5812/cardiovascmed.28952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Seaghdha CM, Hwang SJ, Ho JE, et al. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol. 2013;24:1470–1477. doi: 10.1681/ASN.2012090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal N, Katz R, Seliger S, et al. Galectin-3 and Soluble ST2 and Kidney Function Decline in Older Adults: The Cardiovascular Health Study (CHS) Am J Kidney Dis. 2016;67:994–996. doi: 10.1053/j.ajkd.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papaspyridonos M, McNeill E, de Bono JP, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. 2008;28:433–440. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 13.Aksan G, Gedikli O, Keskin K, et al. Is galectin-3 a biomarker, a player-or both-in the presence of coronary atherosclerosis? J Investig Med. 2016;64:764–770. doi: 10.1136/jim-2015-000041. [DOI] [PubMed] [Google Scholar]
- 14.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 15.Azibani F, Fazal L, Chatziantoniou C, et al. Aldosterone mediates cardiac fibrosis in the setting of hypertension. Curr Hypertens Rep. 2013;15:395–400. doi: 10.1007/s11906-013-0354-3. [DOI] [PubMed] [Google Scholar]
- 16.Lin YH, Chou CH, Wu XM, et al. Aldosterone induced galectin-3 secretion in vitro and in vivo: from cells to humans. PLoS One. 2014;9:e95254. doi: 10.1371/journal.pone.0095254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010;7:1–8. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenay AR, Yu L, van der Velde AR, et al. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am J Physiol Renal Physiol. 2015;308:F500–509. doi: 10.1152/ajprenal.00461.2014. [DOI] [PubMed] [Google Scholar]
- 19.Streetly MJ, Maharaj L, Joel S, et al. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolatsi-Joannou M, Price KL, Winyard PJ, et al. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahsai AW, Cui J, Kaniskan HU, et al. Analogs of tetrahydroisoquinoline natural products that inhibit cell migration and target galectin-3 outside of its carbohydrate-binding site. J Biol Chem. 2008;283:24534–24545. doi: 10.1074/jbc.M800006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Martinez E, Ibarrola J, Calvier L, et al. Galectin-3 Blockade Reduces Renal Fibrosis in Two Normotensive Experimental Models of Renal Damage. PLoS One. 2016;11:e0166272. doi: 10.1371/journal.pone.0166272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmedt V, Desmedt S, Delanghe JR, et al. Galectin-3 in Renal Pathology: More Than Just an Innocent Bystander. Am J Nephrol. 2016;43:305–317. doi: 10.1159/000446376. [DOI] [PubMed] [Google Scholar]
- 25.Drechsler C, Delgado G, Wanner C, et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. J Am Soc Nephrol. 2015;26:2213–2221. doi: 10.1681/ASN.2014010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand IS, Rector TS, Kuskowski M, et al. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail. 2013;15:511–518. doi: 10.1093/eurjhf/hfs205. [DOI] [PubMed] [Google Scholar]
- 27.Grams ME, Rebholz CM, McMahon B, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64:214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebholz CM, Crews DC, Grams ME, et al. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am J Kidney Dis. 2016;68:853–861. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebholz CM, Anderson CA, Grams ME, et al. Relationship of the American Heart Association's Impact Goals (Life's Simple 7) With Risk of Chronic Kidney Disease: Results From the Atherosclerosis Risk in Communities (ARIC) Cohort Study. J Am Heart Assoc. 2016;5:e003192. doi: 10.1161/JAHA.116.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebholz CM, Coresh J, Grams ME, et al. Dietary Acid Load and Incident Chronic Kidney Disease: Results from the ARIC Study. Am J Nephrol. 2015;42:427–435. doi: 10.1159/000443746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvin E, Rawlings AM, Grams M, et al. Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem. 2014;60:1409–1418. doi: 10.1373/clinchem.2014.229427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 33.Gaze DC, Prante C, Dreier J, et al. Analytical evaluation of the automated galectin-3 assay on the Abbott ARCHITECT immunoassay instruments. Clin Chem Lab Med. 2014;52:919–926. doi: 10.1515/cclm-2013-0942. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parrinello CM, Grams ME, Couper D, et al. Recalibration of Blood Analytes over 25 Years in the Atherosclerosis Risk in Communities Study: Impact of Recalibration on Chronic Kidney Disease Prevalence and Incidence. Clin Chem. 2015;61:938–947. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118:496–500. [PubMed] [Google Scholar]
- 37.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 38.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an endpoint for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Collins AJ, Foley RN, Gilbertson DT, et al. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 2015;5:2–7. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebholz CM, Coresh J, Ballew SH, et al. Kidney Failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: Comparing Ascertainment of Treated and Untreated Kidney Failure in a Cohort Study. Am J Kidney Dis. 2015;66:231–239. doi: 10.1053/j.ajkd.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grubb A, Blirup-Jensen S, Lindstrom V, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 43.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grove ML, Yu B, Cochran BJ, et al. Best practices and joint calling of the HumanExome BeadChip: the CHARGE Consortium. PLoS One. 2013;8:e68095. doi: 10.1371/journal.pone.0068095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou GY. Toward using confidence intervals to compare correlations. Psychol Methods. 2007;12:399–413. doi: 10.1037/1082-989X.12.4.399. [DOI] [PubMed] [Google Scholar]
- 47.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 48.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


