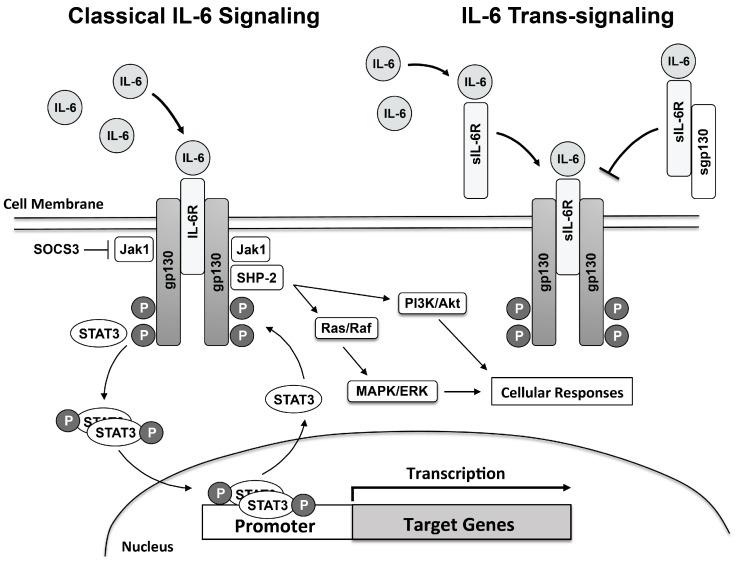
Figure 2.
IL-6 signaling is a function of both classical IL-6 signaling and IL-6-trans-signaling. Classical IL-6 signaling involves IL-6 binding a membrane-bound IL-6 receptor (IL-6R), which facilitates dimerization of membrane-bound gp130. IL-6R lacks intrinsic signaling capability, however activated gp130 contains binding sites for recruitment and phosphorylation of signaling molecules such as Jak/STAT and SHP-2, which play important roles in transcription of IL-6 target genes, such as SOCS3. IL-6-trans-signaling occurs via IL-6 binding soluble IL-6 receptor (sIL-6R), which increases the half-life of IL-6 and serves as an important mechanism of IL-6 signaling in tissues that express gp130 but not IL-6R. Classical IL-6 signaling and IL-6-trans-signaling plays an important role in the diversity of IL-6 signaling. Expression of a soluble form of gp130 (sgp130) functions as an important endogenous inhibitor of IL-6 signaling by binding and preventing IL-6/sIL-6R complex binding and activation of membrane-bound gp130. Jak, Janus-associated Kinase; STAT, Signaling Transducer and Activator of Transcription; SOCS, Suppressor of Cytokine Signaling; PI3K, Phosphatidylinositol-4,5-bisphosphate 3-kinase; Akt, protein kinase B; ERK, Extracellular Signal-Regulated Kinase; MAPK, Mitogen-Activated Protein Kinase.