Fig. 4.
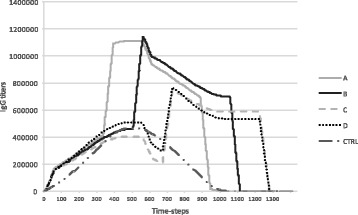
In vivo results. Balb/c mice subdivided in five groups of five individuals were used. The control group (CTRL, the first) received two administration of HPV16L1 at time 0 and 14; The second group (A) is cured with the Neohesperidin at dosage of 10 μg, followed by Neo-hesperidin at dosage of 1 μg, administered, respectively at day 0 and 14. The third group (B) received Neohesperidin, administered at a dosage of 10 μg followed by Neo-hesperidin at dosage of 1 μg, inoculated, respectively at day 0 and 21. The forth group (C) get Neohesperidin at dosage of 10 μg followed by Neo-hesperidin at dosage of 1 μg, administered, respectively at day 0 and 28. The last group (D) is cured with the Neohesperidin at dosage of 10 μg followed by Naringenin at dosage of 1 μg, administered, respectively at day 0 and 28. The total duration of the experiments was 35 days. Subsequent in vivo experiments validated the predictions made by the in silico simulation framework
