Abstract
BACKGROUND:
Non-vitamin K antagonist oral anticoagulants (NOACs) are widely used for prevention of stroke secondary to nonvalvular atrial fibrillation (NVAF). Increased use of NOACs is partially a result of simplified regimens compared with warfarin, which has been associated with poor adherence and persistence to therapy. Few studies have assessed adherence to NOACs, especially using contemporary data now that multiple NOACs are available.
OBJECTIVE:
To evaluate adherence to NOACs in a cohort of newly diagnosed NVAF patients who are commercially insured.
METHODS:
Incident, treatment-naïve NVAF patients were identified in 2013 from a large claims database. Patients were included who initiated rivaroxaban, dabigatran, or apixaban within 30 days after diagnosis. Subjects were required to have 12 months of pre-index information to assess demographic and clinical characteristics (comorbidities, CHA2DS2-VASc, and HAS-BLED scores). Adherence to the index medication and adherence to any oral anticoagulant was assessed using proportion of days covered (PDC) at 3, 6, and 9 months. The number of switches and gaps in therapy were also evaluated. Analyses were stratified by stroke risk scores, and a logistic regression model was used to control for factors that may predict high adherence (PDC ≥ 0.80).
RESULTS:
A total of 3,455 rivaroxaban, 1,264 dabigatran, and 504 apixaban users were included with no major clinical or demographic differences between groups. At 3, 6, and 9 months of follow-up, dabigatran had lower adherence (PDC = 0.77, 0.67, and 0.62) compared with rivaroxaban (PDC = 0.84, 0.75, and 0.70; P < 0.001) and apixaban (PDC = 0.82, 0.75, and 0.71; P < 0.001), as well as nearly twice the number of switches to either other anticoagulants or antiplatelet therapy. At 9 months, 55.0% of rivaroxaban initiators had PDC ≥ 0.80, which was comparable with 56.8% for apixaban and significantly greater than 46.7% for dabigatran (P < 0.001). Adherence was higher overall as stroke risk increased and showed dabigatran had consistently lower adherence compared with the other NOACs. Overall adherence to any oral anticoagulants, allowing for switches to another NOAC or warfarin, was not dependent on the index medication (9-month PDC = 0.74, 0.71, and 0.74 for rivaroxaban, dabigatran, and apixaban initiators). Adjusted analyses showed that increasing age and comorbid hypertension and diabetes were associated with higher adherence. Compared with rivaroxaban, dabigatran initiators had nearly 30% lower odds of being adherent to their index medication, and no differences were observed between apixaban and rivaroxaban. At 9 months, there were no differences between NOACs for overall adherence to oral anticoagulants.
CONCLUSIONS:
In this real-world analysis of adherence to NOACs, rivaroxaban and apixaban had favorable profiles compared with dabigatran, and rivaroxaban appeared to have higher overall adherence among the NOACs. Clinicians and managed care organizations should consider the implications of lower adherence on clinical outcomes as well as quality assessment.
What is already known about this subject
Non-vitamin K antagonist oral anticoagulants (NOACs) are associated with better adherence compared with warfarin therapy.
Adherence has only been compared between single NOACs and warfarin, so little is known about the relative adherence between NOACs, especially now that multiple NOACs are commercially available.
What this study adds
When concurrently compared, there are few differences between patients treated with rivaroxaban, dabigatran, or apixaban.
Rivaroxaban and apixaban had similar adherence at 3, 6, and 9 months after diagnosis, as measured by proportion of days covered, while dabigatran users had significantly lower adherence.
Predictors of overall adherence to oral anticoagulants included older age and comorbid conditions of hypertension and diabetes. Initial therapy choice was not associated with lower adherence but may be detrimental to outcomes because of interruptions in therapy.
Non-vitamin K antagonist oral anticoagulants (NOACs) have seen rapid uptake for stroke prevention in nonvalvular atrial fibrillation (NVAF).1 NOACs include dabigatran, a direct thrombin inhibitor first introduced in 2010, followed by direct factor-Xa inhibitors rivaroxaban in 2011 and apixaban in 2012. Rivaroxaban, dabigatran, and apixaban have been shown to be noninferior to warfarin in efficacy to lower the risk for thromboembolism, as well as for bleed-related safety outcomes.2-4 Edoxaban, a new factor-Xa inhibitor, was also introduced in 2015.5
NOACs have many favorable characteristics compared with warfarin, including fewer drug interactions and dietary restrictions, as well as set dosing regimens. Fewer monitoring and testing requirements are also an arguable advantage of NOAC therapy compared with warfarin.6 Notably, patient and physician preferences typically prefer NOAC profiles compared with warfarin.7 By late 2013, NOACs as a class had overtaken warfarin as the treatment of choice for anticoagulation, and the market share for NOACs is likely to continue increasing.1 Studies comparing the adherence of NOACs with warfarin have mostly shown positive results with NOACs and a tremendous burden of discontinuation with warfarin.8-12 Considering that stroke prevention in NVAF requires prolonged anticoagulation for most patients, the finding that adherence to anticoagulation is higher with NOACs compared with warfarin may support the increased use of NOACs in this patient population.
While adherence to individual NOACs has been compared with warfarin, little is known about the relative adherence for rivaroxaban, dabigatran, and apixaban, especially in the United States.13-15 Despite similarities, these drugs have considerable differences in dosing (once daily dosing for rivaroxaban vs. twice daily for dabigatran and apixaban), as well as varying side-effect profiles—all of which may influence adherence to therapy.6,16-18 Understanding the differences in adherence for each therapy can help inform treatment choices by patients and physicians as well as formulary decision making. In addition, the Pharmacy Quality Alliance (PQA) has recently endorsed a quality measure for managed care plans regarding adherence to NOACs for all indications (NVAF stroke prevention and venous thromboembolism prevention and treatment), potentially making the importance of adherence to NOACs a major consideration for health plans and providers in the near future.13,14,19 The purpose of this study was to evaluate adherence to NOACs in a cohort of newly diagnosed NVAF patients who are commercially insured.
Methods
Cohort Selection and Data Source
This observational cohort study used the Truven Health Analytics MarketScan database. MarketScan data are administrative claims data that includes medical diagnostic and procedural billing information and pharmacy fill records for those with commercial insurance linked to demographic and insurance enrollment information for each individual. The data include medical encounters for nearly 40 million persons each year and are representative of the commercially insured population in the United States. Use of MarketScan data was approved by the University of Kentucky Institutional Review Board.
The goal was to identify treatment-naïve, incident cases of NVAF. Study subjects aged ≥ 18 years were identified who had an incident, inpatient, or outpatient diagnosis of NVAF (International Classification of Diseases, Ninth Revision, Clinical Modification code 427.31) between January 1, 2013, and September 30, 2013. The study dates were chosen to allow assessment of apixaban, which became available in late 2012, making 2013 its first full year on the market. In addition, a minimum of 3 months of follow-up were required to allow sufficient time to assess adherence.
At least 1 additional NVAF diagnosis ≤ 30 days after the index diagnosis was required to avoid rule-out NVAF diagnoses. Subjects were required to have at least 365 days of continuous medical and pharmacy benefits with no previous NVAF diagnosis before the index NVAF diagnosis. Subjects were excluded who had any pre-index claim with an NVAF diagnosis, evidence of oral anticoagulant use, or ≥ 2 international normalized ratio (INR) tests. We also excluded patients with transient AF, mitral stenosis, prosthetic heart valves, hyperthyroidism, or thyrotoxicosis, similar to the requirements of randomized trials and previous observational work.1,20,21 Finally, patients were required to initiate NOAC therapy with rivaroxaban, dabigatran, or apixaban in the 30 days after diagnosis. The final cohort included subjects who had at least 3 months of continuous follow-up time after index, and these subjects were further categorized into subgroups having at least 6 and 9 months of continuous follow-up (Figure 1).
FIGURE 1.
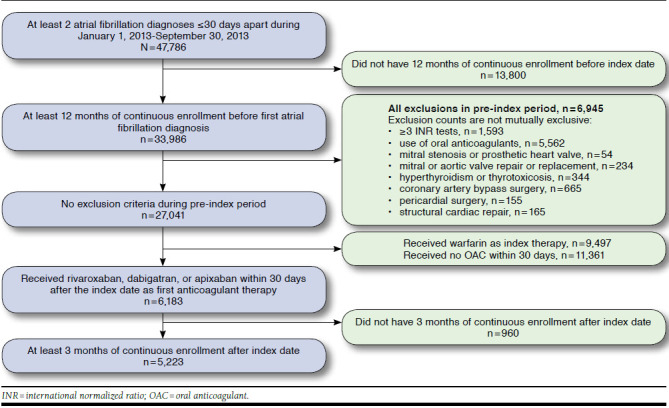
Study Attrition with Inclusion and Exclusion Criteria
Medication Use
Prescription fills for NOACs, warfarin, and antiplatelet medications were observed throughout follow-up using Generic Product Identifier codes (Medi-Span, Indianapolis, IN) linked to National Drug Code (NDC) numbers. Warfarin and anti-platelet use was observed to assess switching behaviors. Because warfarin is widely available through low-cost generic programs and may be absent from pharmaceutical claims data,22-24 we considered INR tests during the follow-up period as a proxy for warfarin fills. This methodology has been shown to have high sensitivity and specificity.25 We considered individuals exposed to warfarin with at least 3 INR tests within the 30 days of follow-up and imputed a days supply of 30 days for each INR test. Antiplatelets included clopidogrel, prasugrel, dipyridamole (± aspirin), and ticlopidine. Prescription aspirin was identified, but it is also widely used as an over-the-counter medication so goes unobserved in claims data with no proxy measures available. Medication use was classified daily for each individual using the date of the prescription fill and the days supply value on the claim. In the presence of overlap of 2 different medications, that is, a switch in therapy, the overlapped portion was credited to the new medication. Where medication overlap was present, only 1 medication’s days supply was included in the adherence calculation for those days so as not to double count. Patients were only classified as treated with antiplatelet medications when an antiplatelet fill was present with no NOAC or warfarin overlap.
Adherence Measures
Proportion of days covered (PDC) was calculated for each individual for the first NOAC medication used after diagnosis (the index medication), as well as for any oral anticoagulant (OAC) used after diagnosis, including the index medication and any OACs that the patient may switch to. PDC is bound between 0 and 1 and is the raw proportion of days that medication is on hand determined by the date the prescription was filled and the days supplied. The interval for PDC assessment started on the day of the first prescription fill, plus the length of the time interval. Time intervals for measurement included 3 months (90 days), 6 months (180 days), and 9 months (270 days). For example, a patient initiating a NOAC on day 10 after diagnosis would be followed up to days 100, 190, and 280. The PDC is defined as the number of days that the index medication was on hand during the time period divided by the total number of days in the time period.
The PDC for use of any OAC (warfarin or another NOAC) during the period was also calculated, which accounts for therapy switching during follow-up. That is, if the same example subject had the index NOAC from day 10-39, and then filled warfarin for days 40-100, PDC for the index medication would be 0.33 (30 days/90 days), and PDC for oral anticoagulation throughout the period would be 1.0. If no NOAC or warfarin was available on a particular day, that day was flagged as having no therapy. PDC was evaluated as the mean PDC for each group, as well as divided into categories of high adherence (≥ 0.80), moderate adherence (0.50-0.79), and low adherence (< 0.50). Medication gaps and switches were also observed. A gap was defined when no observed OAC therapy was present, either no treatment or only antiplatelet therapy. While gaps are also inherent in the PDC calculation, gaps of at least 15 days, at least 30 days, and at least 60 days were noted for each individual.
Study Variables
Demographics, including age, gender, region, and rural/urban residence, were assessed on the index date. Baseline stroke and bleed risk scores were calculated based on the 365-day pre-index period. Stroke risk was assessed using the CHA2DS2-VASc score. 26 CHA2DS2-VASc was stratified by low (0 points), moderate (1 point), high (2-3 points), and very high (≥ 4 points) stroke risk and included 1 point each for heart failure, hypertension, age 65-74 years, diabetes, peripheral vascular disease, and female gender and 2 points each for history of stroke and age ≥ 75 years. The HAS-BLED score was used for bleed risk and included 1 point each for hypertension, renal disease, liver disease, stroke history, prior bleeding, age ≥ 65 years, antiplatelet or nonsteroidal anti-inflammatory drug use, and alcohol or drug use and was stratified as low risk (score = 0), moderate risk (score = 1), and high risk (score ≥ 2).27 HAS-BLED includes a point for labile INR tests, which is not observable in the dataset but would not be important for treatment-naïve, incident cases. A Charlson Comoribidity Index score was also computed for each subject and summarized as the sum of the weighted score.28
Statistical Analyses
Treatment group demographic and clinical characteristics were compared using analysis of variance and chi-squared or Fisher’s exact tests for continuous and categorical variables, respectively. Multiple logistic regression models were estimated including all specified baseline variables and the treatment group assignment predicting the probability of having a high adherence (PDC ≥ 0.80) to NOACs or any oral anticoagulant at 3, 6, and 9 months of follow-up. Odds ratios and 95% confidence intervals are reported from this model. Operationalized definitions of all diagnosis, procedure, and medication codes are included in the Appendix (available in online article). All analyses were conducted using SAS Enterprise Guide, version 7.1 (SAS Institute, Cary, NC). This manuscript was drafted according to STROBE guidelines for observational studies (http://strobe-statement.org/).
Results
Comparison of Treatment Groups
After inclusion/exclusion, 3,455 rivaroxaban, 1,264 dabigatran, and 504 apixaban users were identified during the study period who had at least 3 months of continuous follow-up after NVAF diagnosis. Table 1 shows the baseline characteristics of each treatment groups, as well as the number of patients remaining in the cohort at 6 and 9 months of follow-up. There were some differences between the populations, including nonsignificant counts of comorbidities, gender, and rural/urban status, as well as no significant differences in CHA2DS2-VASc or HAS-BLED scores. Age differences were observed with apixaban having an average of 2 years higher age compared with rivaroxaban and almost 4 years higher age than dabigatran users, driven by a higher proportion of users aged ≥ 75 years. The distribution of region among treatment groups was also different. Much higher proportions of rivaroxaban and dabigatran users were continuously enrolled at 6 and 9 months compared with apixaban. Demographic characteristics were observed for those remaining in the cohort at 6 and 9 months, and similar distributions were observed with no significant differences for these subgroups as well.
TABLE 1.
Characteristics of Study Population for Those Initiating NOACs
| Characteristics | Rivaroxaban n = 3,455 | Dabigatran n = 1,264 | Apixaban n = 504 | |||
|---|---|---|---|---|---|---|
| n | %/SD | n | %/SD | n | %/SD | |
| Age,a mean, SD | 68.1 | 12.4 | 66.5 | 12.3 | 70.3 | 12.2 |
| <65 years | 1,428 | 41.3 | 567 | 44.9 | 168 | 33.3 |
| 65 -74 years | 847 | 24.5 | 333 | 26.3 | 139 | 27.6 |
| ≥75 years | 1,180 | 34.2 | 364 | 28.8 | 197 | 39.1 |
| Gender | ||||||
| Female | 2,051 | 59.4 | 793 | 62.7 | 291 | 57.7 |
| Male | 1,404 | 40.6 | 471 | 37.3 | 213 | 42.3 |
| Regiona | ||||||
| Northeast | 712 | 20.6 | 394 | 31.2 | 89 | 17.7 |
| North Central | 977 | 28.3 | 363 | 28.7 | 152 | 30.2 |
| South | 1,224 | 35.4 | 342 | 27.1 | 182 | 36.1 |
| West | 502 | 14.5 | 155 | 12.3 | 76 | 15.1 |
| Unknown | 40 | 1.2 | 10 | 0.8 | 5 | 1.0 |
| Metro statistical area | ||||||
| Rural | 529 | 15.3 | 221 | 17.5 | 79 | 15.7 |
| Urban | 2,926 | 84.7 | 1,043 | 82.5 | 425 | 84.3 |
| Comoribidities | ||||||
| Hypertension | 1,789 | 51.8 | 668 | 52.8 | 249 | 49.4 |
| Hyperlipidemia | 1,313 | 38.0 | 499 | 39.5 | 196 | 38.9 |
| Heart failure | 197 | 5.7 | 64 | 5.1 | 31 | 6.2 |
| Diabetes | 803 | 23.2 | 317 | 25.1 | 111 | 22.0 |
| Stroke | 209 | 6.0 | 87 | 6.9 | 38 | 7.5 |
| Bleeding | 198 | 5.7 | 59 | 4.7 | 26 | 5.2 |
| Anemia | 231 | 6.7 | 82 | 6.5 | 36 | 7.1 |
| Liver disease | 83 | 2.4 | 28 | 2.2 | 11 | 2.2 |
| Renal disease | 136 | 3.9 | 51 | 4.0 | 19 | 3.8 |
| Vascular disease | 699 | 20.2 | 234 | 18.5 | 105 | 20.8 |
| Dementia | 12 | 0.3 | 7 | 0.6 | 0 | 0 |
| Chronic pulmonary disease | 455 | 13.2 | 150 | 11.9 | 49 | 9.7 |
| Rheumatic disease | 81 | 2.3 | 31 | 2.5 | 11 | 2.2 |
| Peptic ulcer disease | 16 | 0.5 | 5 | 0.4 | 2 | 0.4 |
| Diabetes with complications | 198 | 5.7 | 71 | 5.6 | 30 | 6.0 |
| Cancer | 463 | 13.4 | 151 | 11.9 | 67 | 13.3 |
| Metastatic cancer | 35 | 1.0 | 4 | 0.3 | 2 | 0.4 |
| Charlson Comorbidity Index, mean, SD | 1.1 | 1.6 | 1.1 | 1.5 | 1.1 | 1.5 |
| CHA2DS2-VASc stroke risk score, mean, SD | 2.5 | 1.7 | 2.4 | 1.7 | 2.6 | 1.7 |
| Low (score = 1) | 416 | 12.0 | 178 | 14.1 | 45 | 8.9 |
| Medium (score = 1) | 695 | 20.1 | 255 | 20.2 | 89 | 17.7 |
| High (score = 2-3) | 707 | 20.5 | 259 | 20.5 | 117 | 23.2 |
| Very high (score ≥ 4) | 889 | 25.7 | 311 | 24.6 | 136 | 27.0 |
| HAS-BLED bleed risk score, mean, SD | 1.5 | 1.0 | 1.4 | 1.0 | 1.5 | 1.0 |
| Low (score = 0) | 581 | 16.8 | 230 | 18.2 | 68 | 13.5 |
| Medium (score = 1-2) | 2,389 | 69.1 | 846 | 66.9 | 374 | 74.2 |
| High (score ≥ 3) | 485 | 14.0 | 188 | 14.9 | 62 | 12.3 |
| Enrollment period | ||||||
| 6 months continuous | 2,694 | 78.0 | 1,046 | 82.8 | 325 | 64.5 |
| 9 months continuous | 1,524 | 44.1 | 662 | 52.4 | 111 | 22.0 |
a P < 0.05.
NOAC = non-vitamin K antagonist oral anticoagulation; SD = standard deviation.
Adherence to Treatment
At 3 months of follow-up after treatment initiation, the mean PDC was 0.84, 0.77, and 0.82 for rivaroxaban, dabigatran, and apixaban (P < 0.001). Rivaroxaban users had the highest proportion (73%) of users with high adherence (PDC ≥ 0.80). Dabigatran and apixaban users tended to have more gaps in therapy compared with the rivaroxaban group. Dabigatran users also had 2-3 times the proportion of switchers to another OAC (14.2%). Similar patterns were observed at 6 months of follow-up. Rivaroxaban and apixaban had equivalent PDCs (0.75), while dabigatran users had significantly lower average PDCs (0.67; P < 0.001), as well as a higher proportion of users who switched to another OAC (18.6%). After 9 months, adherence to rivaroxaban (0.70) and apixaban (0.71) were both significantly higher than dabigatran (0.62), and more than half of all rivaroxaban (55.0%) and apixaban users (56.8%) had high adherence compared with 46.7% of dabigatran users (P < 0.001). Among all observed OAC switches for rivaroxaban, 64.7% were to warfarin; 17.0% were to dabigatran; and 18.3% were to apixaban. For dabigatran, 40.5% of all OAC switches were to warfarin; 47.7% were to rivaroxaban; and 11.8% were to apixaban. Apixaban switches included 42.4% to warfarin, 48.5% to rivaroxaban, and 9.1% to dabigatran (Table 2).
TABLE 2.
Adherence Comparison Between Rivaroxaban, Dabigatran, and Apixaban Users at 3, 6, and 9 Months of Follow-up
| Rivaroxaban | Dabigatran | Apixaban | P Value | |
|---|---|---|---|---|
| 3 Months | ||||
| PDC,a mean (SD) | 0.84 (0.24) | 0.77 (0.28) | 0.82 (0.26) | < 0.001 |
| ≥ 0.80, n (%) | 2,521 (73.0) | 783 (62.0) | 354 (70.2) | < 0.001 |
| 0.50-0.79, n (%) | 424 (12.3) | 202 (16.0) | 67 (13.3) | |
| < 0.50, n (%) | 510 (14.8) | 279 (22.1) | 83 (16.5) | |
| Gaps,b n (%) | ||||
| ≥ 15 days | 336 (9.7) | 170 (13.5) | 72 (14.3) | < 0.001 |
| ≥ 30 days | 123 (3.6) | 61 (4.8) | 33 (6.6) | < 0.050 |
| ≥ 60 days | 18 (0.5) | 8 (0.6) | 7 (1.4) | 0.123 |
| Switch,c n (%) | ||||
| Other OAC | 198 (5.7) | 179 (14.2) | 32 (6.4) | < 0.001 |
| Antiplatelet | 59 (1.7) | 31 (2.5) | 6 (1.2) | 0.131 |
| 6 Months | ||||
| PDC,a mean (SD) | 0.75 (0.31) | 0.67 (0.33) | 0.75 (0.29) | < 0.001 |
| ≥ 0.80, n (%) | 1,711 (63.5) | 561 (53.6) | 201 (61.9) | < 0.001 |
| 0.50-0.79, n (%) | 429 (15.9) | 173 (16.5) | 63 (19.4) | |
| < 0.50, n (%) | 554 (20.6) | 312 (29.8) | 61 (18.8) | |
| Gaps,b n (%) | ||||
| ≥ 15 days | 483 (17.9) | 268 (25.6) | 102 (31.4) | < 0.001 |
| ≥30 days | 252 (9.4) | 128 (12.2) | 62 (19.1) | < 0.001 |
| ≥ 60 days | 84 (3.1) | 51 (4.9) | 19 (5.9) | < 0.050 |
| Switch,c n (%) | ||||
| Other OAC | 224 (8.3) | 195 (18.6) | 30 (6.7) | < 0.001 |
| Antiplatelet | 67 (2.5) | 40 (3.8) | 8 (2.5) | 0.232 |
| 9 Months | ||||
| PDC,a mean (SD) | 0.70 (0.34) | 0.62 (0.35) | 0.71 (0.32) | < 0.001 |
| ≥0.80, n (%) | 838 (55.0) | 309 (46.7) | 63 (56.8) | < 0.001 |
| 0.50-0.79, n (%) | 229 (15.0) | 97 (14.7) | 21 (18.9) | |
| < 0.50, n (%) | 457 (30.0) | 256 (38.7) | 27 (24.3) | |
| Gaps,b n (%) | ||||
| ≥ 15 days | 366 (24.0) | 225 (34.0) | 45 (40.5) | < 0.001 |
| ≥ 30 days | 220 (14.4) | 124 (18.7) | 27 (7.3) | < 0.050 |
| ≥ 60 days | 106 (7.0) | 60 (9.1) | 14 (12.6) | < 0.050 |
| Switch,c n (%) | ||||
| Other OAC | 153 (10.0) | 129 (19.5) | 12 (10.8) | < 0.001 |
| Antiplatelet | 48 (3.2) | 29 (4.4) | 4 (3.6) | 0.254 |
a PDC = (sum of days supplied from initiation of therapy + 90, 180, or 270 days) divided by time period length (90, 180, or 270 days). Prescription fills were corrected for early refills and credited to the numerator.
b Gaps are any period where no treatments (OAC or antiplatelet) are observed. Gap lengths (15, 30, 60 days) are not mutually exclusive.
c Individuals who had a switch or a gap that reinitiated the index medication after the gap or switch and within the time period.
PDC = proportion of days covered; OAC = oral anticoagulant; SD = standard deviation.
Adherence was also evaluated among those patients with high and very high stroke risk scores, since guidelines strongly recommend OAC thromboprophylaxis for these subgroups (Table 3).29,30 Adherence was marginally higher among these patients with comparable adherence to rivaroxaban and apixaban and lower adherence observed for dabigatran. Adherence to any OAC over the time period was also evaluated for the overall cohort as well as stratified by stroke risk scores (Table 4). Adherence increased across all treatment groups with increasing stroke risk score and was higher than adherence, considering only index medications. No significant differences were observed between treatment groups for this adherence measure.
TABLE 3.
Adherence Comparison to Index Medication Among Those with High and Very High Stroke Risk
| Rivaroxaban | Dabigatran | Apixaban | P Value | |
|---|---|---|---|---|
| 3 Months | ||||
| CHA2DS2-VASc = 2 or 3, n (%) | ||||
| PDC,a mean (SD) | 0.86 (0.24) | 0.79 (0.27) | 0.79 (0.28) | < 0.050 |
| ≥ 0.80 | 546 (77.2) | 165 (63.7) | 73 (62.4) | < 0.001 |
| 0.50-0.79 | 68 (9.6) | 48 (18.5) | 21 (18.0) | |
| < 0.50 | 93 (13.2 | 46 (17.8) | 23 (19.7) | |
| CHA2DS2-VASc ≥ 4, n (%) | ||||
| PDC,a mean (SD) | 0.87 (0.23) | 0.78 (0.30) | 0.87 (0.23) | < 0.001 |
| ≥ 0.80 | 690 (77.6) | 202 (65.0) | 102 (75.0) | < 0.001 |
| 0.50-0.79 | 104 (11.7) | 40 (12.9) | 21 (15.4) | |
| < 0.50 | 95 (10.7) | 69 (22.2) | 13 (9.6) | |
| 6 Months | ||||
| CHA2DS2-VASc = 2 or 3, n (%) | ||||
| PDC,a mean (SD) | 0.78 (0.30) | 0.68 (0.32) | 0.71 (0.31) | < 0.001 |
| ≥ 0.80 | 370 (68.8) | 111 (52.4) | 42 (56.) | < 0.001 |
| 0.50-0.79 | 73 (13.6) | 41 (19.3) | 17 (22.7) | |
| < 0.50 | 95 (17.7) | 60 (28.3) | 16 (21.3) | |
| CHA2DS2-VASc>4, n (%) | ||||
| PDC,a mean (SD) | 0.80 (0.29) | 0.69 (0.34) | 0.79 (0.26) | < 0.001 |
| ≥ 0.80 | 514 (69.1) | 157 (57.3) | 57 (65.5) | < 0.050 |
| 0.50-0.79 | 111 (14.9) | 46 (16.8) | 18 (20.7) | |
| < 0.50 | 119 (16.0) | 71 (16.8) | 12 (13.8) | |
| 9 Months | ||||
| CHA2DS2-VASc = 2 or 3, n (%) | ||||
| PDC,a mean (SD) | 0.72 (0.33) | 0.65 (0.33) | 0.69 (0.35) | 0.154 |
| ≥ 0.80 | 172 (58.3) | 67 (48.2) | 14 (56.0) | 0.108 |
| 0.50-0.79 | 44 (14.9) | 28 (20.1) | 5 (20.0) | |
| < 0.50 | 79 (26.8) | 44 (31.7) | 6 (24.0) | |
| CHA2DS2-VASc ≥ 4, n (%) | ||||
| PDC,a mean (SD) | 0.75 (0.32) | 0.66 (0.35) | 0.73 (0.31) | < 0.050 |
| ≥ 0.80 | 280 (61.5) | 90 (51.4) | 20 (60.6) | 0.145 |
| 0.50-0.79 | 66 (14.5) | 26 (26.8) | 5 (15.2) | |
| < 0.50 | 109 (24.0) | 59 (33.7) | 8 (24.2) | |
a PDC = (sum of days supply from initiation of therapy + 90, 180, or 270 days) divided by time period length (90, 180, or 270 days). Prescription fills were corrected for early refills and credited to the numerator.
PDC = proportion of days covered; SD = standard deviation.
TABLE 4.
PDC by Any Oral Anticoagulant for Each Treatment Group Stratified by Stroke Risk Scores
| PDC Oral Anticoagulants,a Mean (SD) | Rivaroxaban | Dabigatran | Apixaban |
|---|---|---|---|
| 3 Months | |||
| Overall | 0.86 (0.23) | 0.83 (0.23) | 0.85 (0.23) |
| CHA2DS2-VASc = 0 | 0.79 (0.27) | 0.77 (0.26) | 0.77 (0.27) |
| CHA2DS2-VASc = 1 | 0.81 (0.25) | 0.79 (0.25) | 0.85 (0.23) |
| CHA2DS2-VASc = 2 or 3 | 0.89 (0.21) | 0.86 (0.22) | 0.86 (0.22) |
| CHA2DS2-VASc ≥ 4 | 0.90 (0.12) | 0.87 (0.22) | 0.88 (0.20) |
| 6 Months | |||
| Overall | 0.79 (0.28) | 0.75 (0.29) | 0.79 (0.26) |
| CHA2DS2-VASc = 0 | 0.68 (0.33) | 0.63 (0.33) | 0.70 (0.31) |
| CHA2DS2-VASc = 1 | 0.72 (0.32) | 0.70 (0.31) | 0.77 (0.27) |
| CHA2DS2-VASc = 2 or 3 | 0.82 (0.26) | 0.79 (0.26) | 0.80 (0.25) |
| CHA2DS2-VASc ≥ 4 | 0.84 (0.24) | 0.80 (0.26) | 0.81 (0.23) |
| 9 Months | |||
| Overall | 0.74 (0.32) | 0.71 (0.32) | 0.74 (0.28) |
| CHA2DS2-VASc = 0 | 0.64 (0.35) | 0.56 (0.36) | 0.59 (0.35) |
| CHA2DS2-VASc = 1 | 0.65 (0.34) | 0.63 (0.34) | 0.74 (0.22) |
| CHA2DS2-VASc = 2 or 3 | 0.78 (0.29) | 0.76 (0.28) | 0.76 (0.29) |
| CHA2DS2-VASc ≥ 4 | 0.80 (0.28) | 0.77 (0.29) | 0.74 (0.30) |
Note: There were no statistical differences in any comparison; P values are omitted.
a PDC = (sum of days supplied from initiation of therapy + 90, 180, or 270 days) divided by time period length (90, 180, or 270 days). Prescription fills were corrected for early refills and credited to the numerator. A day was considered to have anticoagulant therapy if warfarin, rivaroxaban, dabigatran, or apixaban was on hand for that day.
PDC = proportion of days covered; SD = standard deviation.
Regression Results
Multiple logistic models were used to control for any residual differences between the treatment groups at each follow-up interval. Baseline characteristics along with treatment group were included in the model predicting the probability of having high adherence to the index medication and to all OACs (PDC ≥ 0.80; Table 5). Individual items of the stroke and bleed risk scores were included separately as individual covariates so that their effects would not be absorbed by the composite scores.
TABLE 5.
Logistic Regression Results Predicting High Adherence
| 3 Months | 6 Months | 9 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CL | OR | 95% CL | OR | 95% CL | ||||
| PDC ≥ 0.80 to index NOAC | |||||||||
| Dabigatran vs. rivaroxaban | 0.60 | 0.53 | 0.70 | 0.66 | 0.57 | 0.77 | 0.72 | 0.60 | 0.87 |
| Dabigatran vs. apixaban | 0.73 | 0.69 | 0.79 | 0.75 | 0.69 | 0.83 | 0.70 | 0.57 | 0.87 |
| Apixaban vs. rivaroxaban | 0.82 | 0.67 | 1.01 | 0.88 | 0.69 | 1.12 | 1.03 | 0.69 | 1.53 |
| Age 65-74 vs. age < 65 | 1.78 | 1.52 | 2.09 | 1.51 | 1.28 | 1.78 | 1.52 | 1.23 | 1.89 |
| Age ≥ 75 vs. age < 65 | 1.76 | 1.51 | 2.05 | 1.84 | 1.56 | 2.17 | 1.81 | 1.46 | 2.26 |
| Hypertension | 1.19 | 1.05 | 1.36 | 1.20 | 1.05 | 1.38 | 1.31 | 1.09 | 1.57 |
| Diabetes | 1.32 | 1.08 | 1.62 | 1.53 | 1.24 | 1.90 | 1.46 | 1.11 | 1.92 |
| PDC ≥0.80 to all OAC | |||||||||
| Dabigatran vs. rivaroxaban | 0.75 | 0.65 | 0.87 | 0.81 | 0.70 | 0.95 | 0.85 | 0.70 | 1.03 |
| Dabigatran vs. apixaban | 0.88 | 0.82 | 0.94 | 0.93 | 0.86 | 1.03 | 0.94 | 0.90 | 1.15 |
| Apixaban vs. rivaroxaban | 0.85 | 0.69 | 1.06 | 0.87 | 0.68 | 1.11 | 0.90 | 0.61 | 1.35 |
| Age 65-74 vs. age < 65 | 1.98 | 1.68 | 2.34 | 1.68 | 1.41 | 1.99 | 1.65 | 1.33 | 2.05 |
| Age ≥ 75 vs. age < 65 | 1.92 | 1.63 | 2.26 | 1.92 | 1.62 | 2.27 | 1.93 | 1.55 | 2.40 |
| Hypertension | 1.20 | 1.05 | 1.38 | 1.18 | 1.03 | 1.37 | 1.32 | 1.10 | 1.59 |
| Diabetes | 1.31 | 1.06 | 1.62 | 1.48 | 1.18 | 1.85 | 1.32 | 1.00 | 1.75 |
Note: Other variables included in the model with nonsignificant effects: gender, urbal/rural status, region, Charlson comorbidity score, history of stroke, vascular disease, renal disease, liver disease, prior bleeding or medications that increase risk of bleeding, alcohol abuse, hyperlipidemia, cancer ± metastases, and dementia. Bold results are significant at P < 0.05.
CL = confidence limit; NOAC = non-vitamin K antagonist oral anticoagulant; OAC = oral anticoagulant; OR = odds ratio; PDC = proportion of days covered.
The primary comparison was the effect of treatment group assignment on adherence. As shown in uncontrolled analyses, rivaroxaban users were consistently more adherent to their index therapies. This trend persisted for OAC adherence until 9 months of follow-up. Comparisons between apixaban and rivaroxaban patients produced point estimates showing an advantage with rivaroxaban but with confidence limits crossing the null. Increasing age was a consistent significant predictor of high adherence, increasing the odds of adherence among those users aged 65-74 years and ≥ 75 years by roughly 50%-100%, compared with those patients aged < 65 years. Presence of a history of hypertension or diabetes also increased the odds of high adherence.
Discussion
Among the commercially insured NVAF patients in the United States, this study found consistently lower adherence for dabigatran compared with rivaroxaban and apixaban. These findings persisted for the overall cohort as well as subgroups of patients at high and very high risk of stroke according to baseline CHA2DS2-VASc scores. Using a cutoff of PDC ≥ 0.80 to distinguish adherent individuals, this translated into an absolute difference of roughly 10% fewer dabigatran users meeting this cutpoint compared with either rivaroxaban or apixaban users. Differences between rivaroxaban and apixaban existed but were either not consistent or not significant in all analyses. These results were confirmed in fully adjusted analyses showing consistently lower adherence with dabigatran that the 2 other NOACs, and no difference between apixaban and rivaroxaban.
The decrease in adherence to dabigatran was also observed in the analysis of adherence to any OAC over the treatment periods. This measure is most important among those patients with CHA2DS2-VASc ≥ 2, since guidelines routinely recommend long-term OAC for stroke prevention.29,30 Measuring PDC for any OAC takes into account switching between NOACs or warfarin and serves as a proxy measure of adherence to treatment guidelines. These results further showed that rivaroxaban and apixaban treatment groups did not differ in OAC adherence, while dabigatran users tended to have lower adherence compared with these groups. These findings suggest that initiation of dabigatran as initial treatment can impact long-term treatment adherence, even when allowing for switching. More research is needed to understand this effect and to systematically assess the outcomes associated with these findings.
Adherence and/or persistence to NOACs has mostly been assessed for single products or compared with warfarin, and few studies have assessed these measures in the United States.8,10-13,31,32 Existing studies have mostly focused on dabigatran, since it was first to market, and rivaroxaban and have shown similar results compared with our study. Like this study, adherence to therapy was associated with increased age as well as increased stroke risk.9,10 However, few of these studies have concurrently compared adherence of dabigatran, rivaroxaban, and apixaban among patients with NVAF. Although our findings are consistent with these previous studies, it was important to consider adherence and switching concurrently for NOACs now that multiple treatment options are available.
A recent study by Crivera et al. (2015) assessed PDC to NOACs in a large managed care database and similarly categorized adherent patients as those with PDC ≥ 0.80.14 They found that around 75% of rivaroxaban, 70% of apixaban, and 67% of dabigatran users were highly adherent to therapy. These values are higher than the proportions found in this study and showed a larger difference between rivaroxaban and apixaban. Their methodology used a distinct algorithm for identifying users according to a PQA standardized method and was also not specific to NVAF, since they considered NOAC adherence across all indications. Their findings are consistent with the current study, however, showing dabigatran with lower overall adherence compared with both comparators.
The switching patterns observed in this study suggest some differences in patient and physician preferences. While more dabigatran users switched therapy compared with the other groups, a larger proportion switched to another NOAC (59.5%) instead of warfarin (40.5%). Similarly, 57.6% of apixaban users switched to another NOAC, while a majority of rivaroxaban users switched to warfarin (64.7%). This may be because of the once-daily dosing of rivaroxaban and warfarin, which has been shown to be an important consideration for patients and physicians and may be even more important for patients who tend to be less adherent.7,16 Although dosing regimen may be an important switching consideration, the twice-daily dosing regimen of apixaban did not suggest an effect on adherence.
Multiple factors, including safety and efficacy, as well as the dosing regimen of each treatment, likely contribute to the decision between NOACs, as well as the adherence and switching behaviors observed.7,21 Dabigatran, in particular, has been the subject of multiple adverse events reports, such as the relatively common occurrence of dyspepsia, as well as more rare, but serious, cardiovascular events.33-35 These reports have led to a large reduction in market share for dabigatran among NOACs, despite being the first to market.1 Other unobservable factors may also include patient and physician preferences or pharmacy benefit design (e.g., prior authorizations or step therapy), which may introduce a channeling effect to particular products. Among observed characteristics, including demographic and clinical characteristics, age was the only metric that differed significantly between the treatment groups. This difference suggests that treatment choices are being largely driven by these unobserved factors, since there were virtually no differences between patients.
Adherence to anticoagulation is pivotal to prevent stroke associated with NVAF.36-38 Although overall adherence has not been shown to be associated with stroke risk, gaps in therapy during treatment with warfarin has been shown to impart increased risk of stroke.39 Gaps in therapy can include missed doses, as well as delays in filling subsequent prescriptions. While missed doses could not be assessed in these data, we did observe the number of gaps for each treatment group and showed that rivaroxaban was associated with fewer gaps compared with the other treatments. While we assessed longer gaps of 15, 30, and 60 days, even small gaps or missed doses while on NOACs can be high risk, considering the shorter half-life of these medications.6 Thus, while the choice of initial NOAC did not predict overall adherence to OAC, interruptions in therapy could be detrimental for clinical outcomes.
Adherence to NOACs is poised to become an important policy issue for managed care companies, since the PQA has developed a quality metric for this measure. PQA adherence measures exist for other medication classes for diabetes and hypercholesterolemia and have been incorporated as standardized metrics to compare health plan and physician quality.40 For Medicare Part D and Medicare Advantage plans, these measure are given a significant weight in the star ratings calculation.41 Given that enrollee selection of plans and reimbursement are tied to these star ratings, health plans are strongly motivated to develop interventions to increase their ratings, including enrollee-directed interventions or formulary management decisions.42,43 Further, in practices where payment is tied to provider performance, these measures may also affect prescribers and make this study more important to help guide prescribing choices.
Limitations
This study is subject to the limitations of all claims-based studies.44,45 Notably for this adherence study, the data captured prescription use and assumed that a patient consumed the medication and was compliant with the dosing regimen, although this cannot be confirmed. We did not incorporate patients who initiated warfarin, since it is well understood that warfarin therapy is wrought with high discontinuation rates and poor adherence. Further, previous work currently in review showed that there are more differences between warfarin and NOAC users in the post-NOAC era, which could have potentially biased these results. Further, anecdotal evidence suggests that many physicians prescribe warfarin for more complicated patient cases or for patients who require frequent visits, which would have further biased any results with warfarin. Our patient selection involved 3 time periods of continuous eligibility to be included in the analysis so as to have standardized follow-up times between patients.46 This use of 3 time periods mostly removed those who died during follow-up, as well as those who disenrolled from their health plans. Thus, the measure of adherence will not account for these groups of patients.47 However, previous studies have shown that those who die during initial treatment of NVAF have similar adherence to those who survive.11 We restricted the analysis to the first 9 months of 2013 to allow for sufficient follow-up for calculation of adherence. Finally, this study used a sample of commercially insured individuals, so these results may have limited generalizability in other samples.
Conclusions
In this study of newly diagnosed, treatment-naïve NVAF patients, those initiating anticoagulation with dabigatran had lower adherence and more switching during 3, 6, and 9 months of follow-up compared with rivaroxaban and apixaban users. Overall adherence to any anticoagulation among those with increased stroke risk was also similar for rivaroxaban and apixaban and lower for dabigatran users. Factors associated with adherence in this study included older age and comorbid hypertension and diabetes. Since adherence to NOACs is a potential future quality of care indicator, managed care plans and prescribers should be aware of the variable adherence rates between treatment options.
APPENDIX. Operational Definitions Used to Determine Patient Characteristics
| Condition | Definition |
|---|---|
| Atrial fibrillation | ICD-9-CM diagnosis: 427.31 |
| Coronary artery bypass surgery | ICD-9-CM procedures: 36.10 to 36.19 |
| Pericardial surgery | ICD-9-CM procedure: 37.10 to 37.12, 37.31 to 37.33, or 37.40 |
| Structural cardiac repair | ICD-9-CM procedure: 35.00 to 35.04, 35.31 to 35.39, 35.41 to 35.42, 35.50 to 35.56, 35.60 to 35.63 or 35.70 to 35.73 |
| Mitral stenosis or prosthetic heart valve | ICD-9-CM diagnosis: 394.0, 394.2, 396.0, 396.1, 396.8, V43.3, or V42.2 |
| Mitral or aortic valve repair or replacement | ICD-9-CM procedure: 35.10 to 35.14 or 35.20 to 35.28 |
| Concomitant hyperthyroidism or thyrotoxicosis | ICD-9-CM diagnosis: 242.0 to 242.9 or methimazole or propylthiouracil use (codes below) |
| Prior stroke/transient ischemic attack | ICD-9-CM diagnosis: 433.xx, 434.xx, 435.0, 435.1, 435.2, 435.3, 435.8, 435.9 |
| Congestive heart failure | ICD-9-CM diagnosis: 398.91, 402.01, 402.11, 425.1, 425.4, 425.7, 428.X |
| Diabetes mellitus | ICD-9-CM diagnosis: 250.x |
| Hypertension | ICD-9-CM diagnosis: 401.x, 402.x, 403.x, 404.x, 405 |
| Vascular diseases | ICD-9-CM diagnosis: 410, 411, 412, 413, 414, 440, 441, 442, 443, 444, 445 or Procedure codes: 00.66, 36.0, 36.1, 39.25, 33510-33545, 34051, 34151, 34201, 34203, 34800-34834, 34900, 35081-35103, 35131, 35132, 35141, 35142, 35151, 35152, 35331, 35341, 35351, 35355, 35361, 35363, 35371, 35372, 35381, 35450, 35452, 35454, 35456, 35459, 35470, 35471, 5472, 35473, 35474, 35480, 35481, 35482, 35483, 35485, 35490, 35491, 35492, 35493, 35495, 35521, 35531, 35533, 35541, 35546, 35548, 35549, 35551,35556, 35558, 35563, 35565, 35566, 35571, 35583, 35585, 35587, 35621, 35623, 35646, 35647, 35651, 35654, 35656, 35661, 35663, 35665, 35666, 35671, 92980, 92981, 92982, 92984 |
| Prior bleeding | ICD-9-CM diagnosis: 423.0, 430.xx, 431.xx, 432.xx, 852.0x, 852.2x, 852.4x, 853.0x, 455.2, 455.5, 455.8, 456.0, 456.20, 459.0, 530.7, 530.82, 531.00, 531.01, 531.20, 531.21, 531.40, 531.41, 531.60, 531.61, 533.01, 533.20, 533.21, 533.40, 533.41, 533.60, 533.61, 534.00, 534.01, 534.20, 534.21, 534.40, 534.41, 534.60, 534.61, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 537.83, 562.02, 562.03, 562.12, 562.13, 568.81, 569.3, 569.85, 578, 578.0, 578.1, 578.9, 593.81, 599.7, 719.10, 719.11, 719.12, 719.13, 719.14, 719.15, 719.16, 719.17, 719.18, 719.19, 784.7, 784.8, 786.3 |
| Anemia | ICD-9-CM diagnosis: 285.1, 282.41, 282.42, 282.5, 282.60-282.64, 282.68, 282.69, 280.1, 280.8, 280.9, 281.0, 281.1, 281.2, 281.3, 281.4, 281.8, 281.9, 284.0, 284.01, 284.09, 284.1, 284.8, 284.81, 284.89, 284.9, 280, 283.0, 283.1, 283.11, 283.19, 283.2, 283.9, 282.0, 282.1, 282.2, 282.3, 282.4, 282.49, 282.7, 282.8, 282.9, 284.2, 285.0, 285.21, 285.22, 285.29, 285.8, 285.9 |
| Renal failure | ICD-9-CM diagnosis: 584, 584.5, 584.6, 584.7, 584.8, 584.9, 585, 585.3, 585.4, 585.5, 585.6, 585.9, 586, 792.5, V420, V451, V4511, V4512, V560, V561, V562, V5631, V5632, V568 |
| Hyperlipidemia | ICD-9-CM codes: 272.0x-272.4x |
| Dementia | ICD-9-CM codes: 290.x, 294.1, 331.2 |
| Chronic pulmonary disease | ICD-9-CM codes: 416.8, 416.9, 490.x-505.x, 506.4, 508.1, 508.8 |
| Rheumatism | ICD-9-CM codes: 446.5, 710.0-710.4, 714.0-714.2, 714.8, 725.x |
| Diabetes with complications | ICD -9- CM codes: 250.4-250.7 |
| Cancer | ICD-9-CM codes: 140.x-172.x, 174.x-195.8, 200.x-208.x, 238.6 |
| Metastatic cancer | ICD-9-CM codes: 196.x-199.x |
| Alcohol use | ICD-9-CM diagnosis: 291.0, 291.1, 291.2, 303, 305.0, 535.3, V11.3 |
| Medications | |
| Warfarin | First 6 digits of GPI code: 832000XXXXXXXX |
| Dabigatran | First 6 digits of GPI code: 83337030XXXXXX |
| Rivaroxaban | First 6 digits of GPI code: 83370060XXXXXX |
| Apixaban | First 6 digits of GPI code: 83370010XXXXXX |
| Aspirin | First 6 digits of GPI code: 641000XXXXXXXX |
| Antiplatelets | First 4 digits of GPI code: 8515XXXXXXXXX |
| Methimazole/propylthiouracil | First 6 digits of GPI code: 283000XXXXXXXX |
GPI = Generic Product Identifier; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
References
- 1.Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med. 2014;127(11):1075-82.e1. Available at: http://www.amjmed.com/article/S0002-9343(14)00399-4/pdf. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-62. [DOI] [PubMed] [Google Scholar]
- 3.Adam SS, McDuffie JR, Ortel TL, Williams JW Jr.. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med. 2012;157(11):796-807. [DOI] [PubMed] [Google Scholar]
- 4.Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J.. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5(4):480-86. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3471365/. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan L, Pisano M.. Edoxaban (savaysa): a factor Xa inhibitor. Pharm Ther. 2015;40(10):651-95. [PMC free article] [PubMed] [Google Scholar]
- 6.Rosanio S, Keylani AM, D’Agostino DC, DeLaughter CM, Vitarelli A.. Pharmacology, benefits, unaddressed questions, and pragmatic issues of the newer oral anticoagulants for stroke prophylaxis in non-valvular atrial fibrillation and proposal of a management algorithm. Int J Cardiol. 2014;174(3):471-83. [DOI] [PubMed] [Google Scholar]
- 7.Andrade JG, Krahn AD, Skanes AC, Purdham D, Ciaccia A, Connors S.. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can J Cardiol. 2016. Jun;32(6):747-53. Available at: http://www.onlinecjc.ca/article/S0828-282X(15)01492-0/fulltext. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Laliberte F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30(7):1317-25. [DOI] [PubMed] [Google Scholar]
- 9.Fang MC, Go AS, Chang Y, et al. Warfarin discontinuation after starting warfarin for atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3(6):624-31. Available at: http://circoutcomes.ahajournals.org/content/3/6/624.long. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellucci LA, Shaw J, van der Salm K, et al. Self-reported adherence to anticoagulation and its determinants using the morisky medication adherence scale. Thromb Res. 2015;136(4):727-31. [DOI] [PubMed] [Google Scholar]
- 11.Forslund T, Wettermark B, Hjemdahl P.. Comparison of treatment persistence with different oral anticoagulants in patients with atrial fibrillation. Eur J Clin Pharmacol. 2016;72(3):329-38. [DOI] [PubMed] [Google Scholar]
- 12.Martinez C, Katholing A, Wallenhorst C, Freedman SB.. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost. 2016;115(1):31-39. Available at: https://th.schattauer.de/en/contents/archive/issue/2297/manuscript/24687.html. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 13.McHorney CA, Crivera C, Laliberte F, et al. Adherence to non-vitamin-Kantagonist oral anticoagulant medications based on the Pharmacy Quality Alliance measure. Curr Med Res Opin. 2015;31(12):2167-73. [DOI] [PubMed] [Google Scholar]
- 14.Crivera C, Nelson WW, Bookhart B, et al. Pharmacy Quality Alliance measure: adherence to non-warfarin oral anticoagulant medications. Curr Med Res Opin. 2015;31(10):1889-95. [DOI] [PubMed] [Google Scholar]
- 15.Zalesak M, Siu K, Francis K, et al. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ Cardiovasc Qual Outcomes. 2013;6(5):567-74. Available at: http://circoutcomes.ahajournals.org/content/6/5/567.long. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Vrijens B, Heidbuchel H.. Non-vitamin K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace. 2015;17(4):514-23. Available at: http://europace.oxfordjournals.org/content/17/4/514.long. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Vrijens B, Urquhart J.. From monitoring to vigilance about patient adherence to new oral anticoagulants. Europace. 2014;16(1):149. Available at: http://europace.oxfordjournals.org/content/16/1/149.1.long. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Laliberté F, Nelson WW, Lefebvre P, Schein JR, Rondeau-Leclaire J, Duh MS.. Impact of daily dosing frequency on adherence to chronic medications among non-valvular atrial fibrillation patients. Adv Ther. 2012;29(8):675-90. [DOI] [PubMed] [Google Scholar]
- 19.National Quality Measures Clearinghouse . Proportion of days covered (PDC): percentage of patients who filled at least two prescriptions for a non-warfarin oral anticoagulant on two unique dates of service at least 180 days apart, received greater than 60 days supply of the medication, and who met the PDC threshold of 80% during the measurement period. Measure summary. July 2015. Agency for Healthcare Research and Quality. Rockville, MD. Available at: https://www.qualitymeasures.ahrq.gov/summaries/summary/47502/proportion-of-days-covered-pdc-percentage-of-patients-who-filled-at-least-two-prescriptions-for-a-nonwarfarin-oral-anticoagulant-on-two-unique-dates-of-service-at-least-180-days-apart-received-greater-than-60-days-supply-of-the-medication-and-who-met-the-pdc-t. Accessed September 15, 2016. [Google Scholar]
- 20.Shewale A, Johnson J, Li C, Nelsen D, Martin B.. Variation in anticoagulant recommendations by the guidelines and decision tools among patients with atrial fibrillation. Healthcare. 2015;3(1):130-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G.. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015;115(8):1095-101. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4380530/. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013;22(8):899-906. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4012425/. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauly NJ, Talbert JC, Brown J.. Low-cost generic program use by Medicare beneficiaries: implications for medication exposure misclassification in administrative claims data. J Manag Care Spec Pharm. 2016;22(6):741-51. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2016.22.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauly NJ, Brown JD.. Prevalence of low-cost generic program use in a nationally representative cohort of privately insured adults. J Manag Care Spec Pharm. 2015;21(12):1162-70. Available at: http://www.jmcp.org/doi/full/10.18553/jmcp.2015.21.12.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA.. Atrial fibrillation and stroke in the general Medicare population: a 10-year perspective (1992 to 2002). Stroke. 2006;37(8):1969-74. Available at: http://stroke.ahajournals.org/content/37/8/1969.long. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-72. [DOI] [PubMed] [Google Scholar]
- 27.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY.. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-100. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-39. [DOI] [PubMed] [Google Scholar]
- 29.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S-75S. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3278056/. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-76. Available at: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0735109714017409?returnurl=http:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0735109714017409%3Fshowall%3Dtrue&referrer=http:%2F%2Fwww.ncbi.nlm.nih.gov%2Fpubmed%2F24685669. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Beyer-Westendorf J, Forster K, Ebertz F, et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients-results from the dresden non-interventional oral anticoagulation registry. Europace. 2015;17(4):530-38. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4381834/. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanemaaijer S, Sodihardjo F, Horikx A, et al. Trends in antithrombotic drug use and adherence to non-vitamin K oral anticoagulants in the Netherlands. Int J Clin Pharm. 2015;37(6):1128-35.Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4619456/. Accessed September 15, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol. 2013;11(3):246-52. [DOI] [PubMed] [Google Scholar]
- 34.Artang R, Rome E, Nielsen JD, Vidaillet HJ.. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am J Cardiol. 2013;112(12):1973-79. Available at: http://www.ajconline.org/article/S0002-9149(13)01709-8/abstract. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Mak K. Coronary and mortality risk of novel oral antithrombotic agents: a meta-analysis of large randomised trials. BMJ Open. 2012;2(5):e001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorne K, Jayathissa S, Dee S, et al. Adherence and outcomes of patients prescribed dabigatran (Pradaxa) in routine clinical practice. Intern Med J. 2014;44(3):261-65. [DOI] [PubMed] [Google Scholar]
- 37.Ewen S, Rettig-Ewen V, Mahfoud F, Bohm M, Laufs U.. Drug adherence in patients taking oral anticoagulation therapy. Clin Res Cardiol. 2014;103(3):173-82. [DOI] [PubMed] [Google Scholar]
- 38.Casciano JP, Dotiwala ZJ, Martin BC, Kwong WJ.. The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm. 2013;19(4):302-16. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2013.19.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spivey CA, Liu X, Qiao Y, et al. Stroke associated with discontinuation of warfarin therapy for atrial fibrillation. Curr Med Res Opin. 2015;31(11):2021-29. [DOI] [PubMed] [Google Scholar]
- 40.Pharmacy Quality Alliance . PQA performance measures. 2015. Available at: http://pqaalliance.org/measures/default.asp. Accessed September 15, 2016. [Google Scholar]
- 41.Academy of Managed Care Pharmacy, American Pharmacists Association . Medicare star ratings: stakeholder proceedings on community pharmacy and managed care partnerships in quality. J Am Pharm Assoc (2003). 2014;54(3):228-40. [DOI] [PubMed] [Google Scholar]
- 42.Erickson SC, Leslie RS, Patel BV.. Is there an association between the high-risk medication star ratings and member experience CMS star ratings measures?. J Manag Care Spec Pharm. 2014;20(11):1129-36. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2014.20.11.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid RO, Deb P, Howell BL, Shrank WH.. Association between Medicare advantage plan star ratings and enrollment. JAMA. 2013;309(3):267-74. Available at: http://jama.jamanetwork.com/article.aspx?articleid=1557733. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]
- 44.Schneeweiss S, Avorn J.. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323-37. [DOI] [PubMed] [Google Scholar]
- 45.Zhan C, Miller MR.. Administrative data based patient safety research: a critical review. Qual Saf Health Care. 2003;12(Suppl 2):ii58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou M, Chang HY, Segal JB, Alexander GC, Singh S.. Adherence to a novel oral anticoagulant among patients with atrial fibrillation. J Manag Care Spec Pharm. 2015;21(11):1054-62. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2015.21.11.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorst-Rasmussen A, Skjoth F, Larsen TB, Rasmussen LH, Lip GY, Lane DA.. Dabigatran adherence in atrial fibrillation patients during the first year after diagnosis: a nationwide cohort study. J Thromb Haemost. 2015;13(4):495-504. Available at: http://onlinelibrary.wiley.com/doi/10.1111/jth.12845/abstract. Accessed September 15, 2016. [DOI] [PubMed] [Google Scholar]


