Fig. 1.
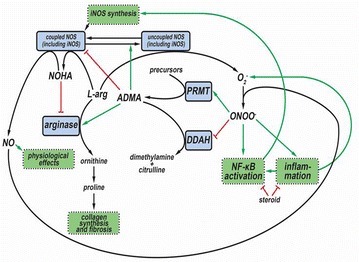
Intertwining of asymmetric dimethylarginine (ADMA) with the nitric-oxide (NO) homeostasis in inflammatory airway diseases. Nitric oxide (NO) is formed from l-arginine through N-hydroxyarginine (NOHA) by NO-synthase enzymes (NOS) including the inducible one (iNOS). In bronchial asthma, nuclear transcription factor NF-κB, activated by inflammatory cytokines, increases the expression of iNOS. This augmented expression promotes formation of NO, which compound, by reacting with superoxide anion (O2−) common under inflammatory conditions, provides peroxynitrite anion (ONOO−) and eventually results in nitrosative stress. This stress increases the production of asymmetric dimethylarginine (ADMA) by enhancing the expression of protein methyltransferase enzymes (PRMT), responsible for ADMA formation, and by decreasing the expression of dimethylargininase (DDAH), which participates in the elimination of ADMA. ADMA is an endogenous inhibitor and, in addition, a natural uncoupler of all NOS isoforms. Uncoupling of NOS leads to the production of O2− instead of NO. Thus, elevated ADMA level, on one hand, decreases NO production, and, on the other hand, increases levels of oxidative and nitrosative agents via uncoupling NOS. Higher ADMA level also results in enhanced arginase activity, thereby contributing to collagen synthesis and (possibly) to the evolution of a (reversible) lung fibrosis. Corticosteroid therapy can prevent the iNOS-related processes elicited by inflammation (via inhibiting inflammatory processes and directly inhibiting the NF-κB expression) that leads to decreased iNOS expression and consequently lower production of NO, ADMA and oxidative/nitrosative agents. Green arrows: activation, enhancement; red lines: inhibition; black arrows: transformation, metabolic connection
