Fig 2. Common characteristics of studies.
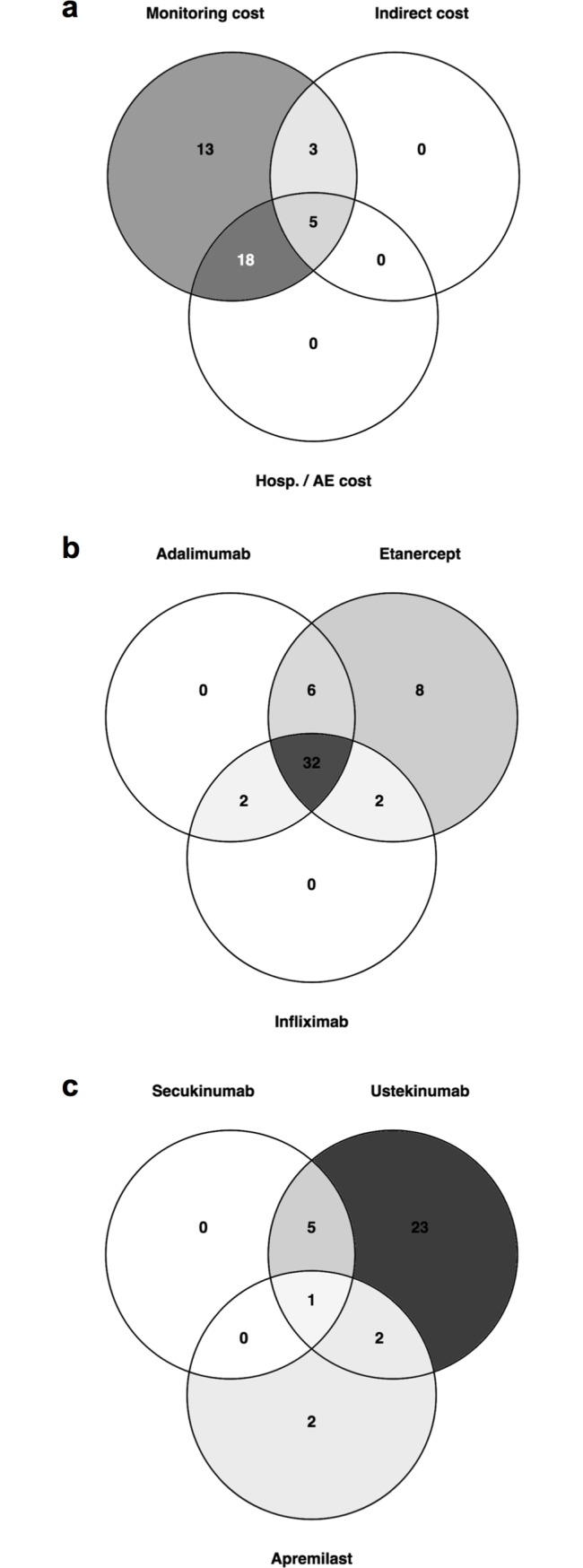
Depicted are the number of studies sharing included cost elements (a), analyses incorporating TNF-inhibitors (b) and studies integrating ustekinumab, secukinumab and apremilast, which were approved more recently (c). AE: adverse events; Hosp: hospitalization.
