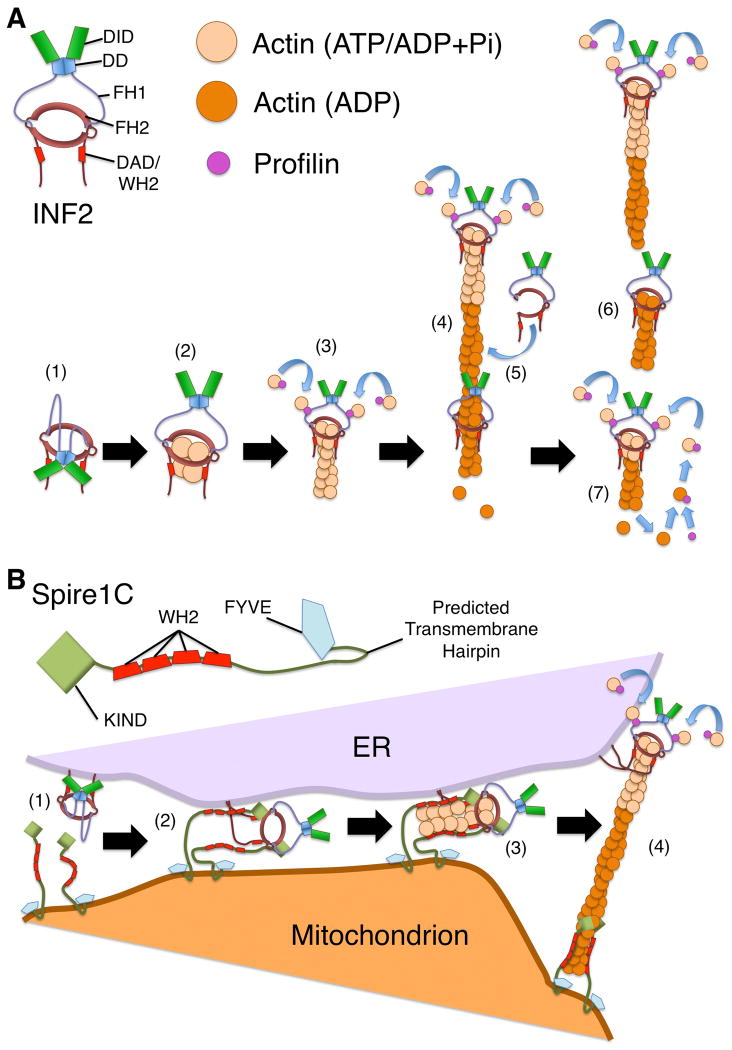
Figure 2. Effects of INF2 on actin filament dynamics.
(A) INF2 stimulates actin polymerization and depolymerization. (1) Interaction between the DID and DAD hold INF2 in an autoinhibited state. (2) With activation of INF2, ATP-actin associates with the FH2 domain and the WH2-like DAD, with a potential ratio of four actin monomers per INF2 dimer [Gurel et al., 2015]. (3) ATP-actin monomers bound to profilin are recruited by INF2 through interaction of profilin with the FH1 domains, followed by transfer of the monomers to the FH2 domain-bound barbed end. (4) As the filament elongates, with INF2 remaining at the growing barbed end, actin hydrolyzes ATP and releases inorganic phosphate, generating ADP-bound F-actin. (5) INF2 associates with ADP-bound actin, possibly through transient opening of the FH2 dimer to encircle the filament. (6) In a WH2/DAD-dependent manner, INF2 promotes filament severing. (7) As ADP-actin depolymerizes, actin monomers bind to profilin, which catalyzes ADP/ATP-nucleotide exchange. This maintains a pool of ATP-bound actin that permits further INF2-stimulated actin polymerization. (B) A model for Spire1C and INF2 cooperation during mitochondrial fission. For mitochondrial fission, INF2 might cooperate with Spire1C to promote actin filament assembly by a “Missile Launcher” type mechanism. (1) The CAAX-form of INF2 associates with the ER membrane through C-terminal prenylation, while Spire1C is anchored to the mitochondrial outer membrane, potentially through a predicted transmembrane hairpin. (2) Analogous to the interaction between Drosophila Spire and CAPU formin, the Spire1C KIND domain binds the INF2 FH2 domain. (3) Actin filaments are nucleated by the Spire1C/INF2 complex in a manner strongly dependent on actin monomer-binding WH2 domains of Spire1C. (4) As the filament elongates, the Spire1C/INF2 interaction is broken, with Spire1C remaining associated near the pointed end at the mitochondrial surface, and INF2 remaining associated with the elongating barbed end at the ER surface.