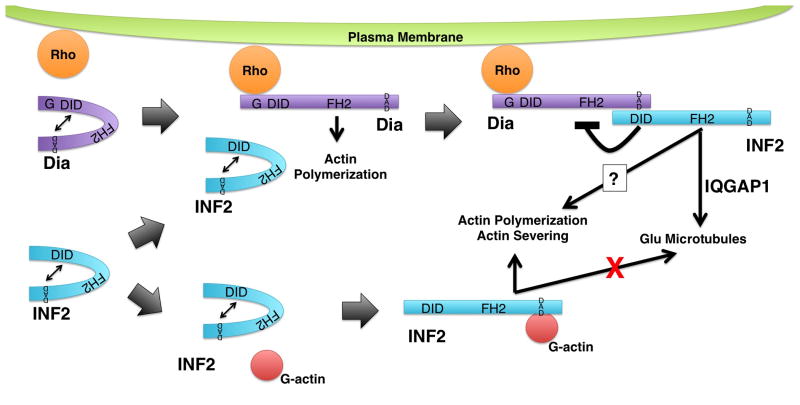
Figure 4. Regulation of INF2 activity.
INF2 (blue) is autoinhibited by interaction of its DID and DAD. Autoinhibition can be relieved through at least two pathways. (Top) One pathway involves mammalian Diaphanous-related formins (Dia, puple). Dia formins are also autoregulated via interaction between their DID and DAD. This autoregulation can be disrupted by binding of a Rho family GTPase, permitting Dia to stimulate actin polymerization. The INF2 DID has greater affinity for the Dia DAD than for the INF2 DAD leading to INF2 DID/Dia DAD interaction. This breaks the INF2 autoinhibition, allowing INF2 to promote the formation of stabilized Glu microtubules in an IQGAP1-dependent manner. Another consequence of the INF2 DID/Dia DAD interaction is the inhibition of Dia-dependent actin polymerization. (Bottom) A second pathway for breaking INF2 autoinhibition is through binding of G-actin to the INF2 DAD. Interaction with G-actin inhibits INF2 from interacting with microtubules, but permits INF2 to interact with actin to promote polymerization, and perhaps severing. Note, INF2 and Dia are depicted as monomers is for visual clarity, only. Both formins are expected to exist as homodimers.