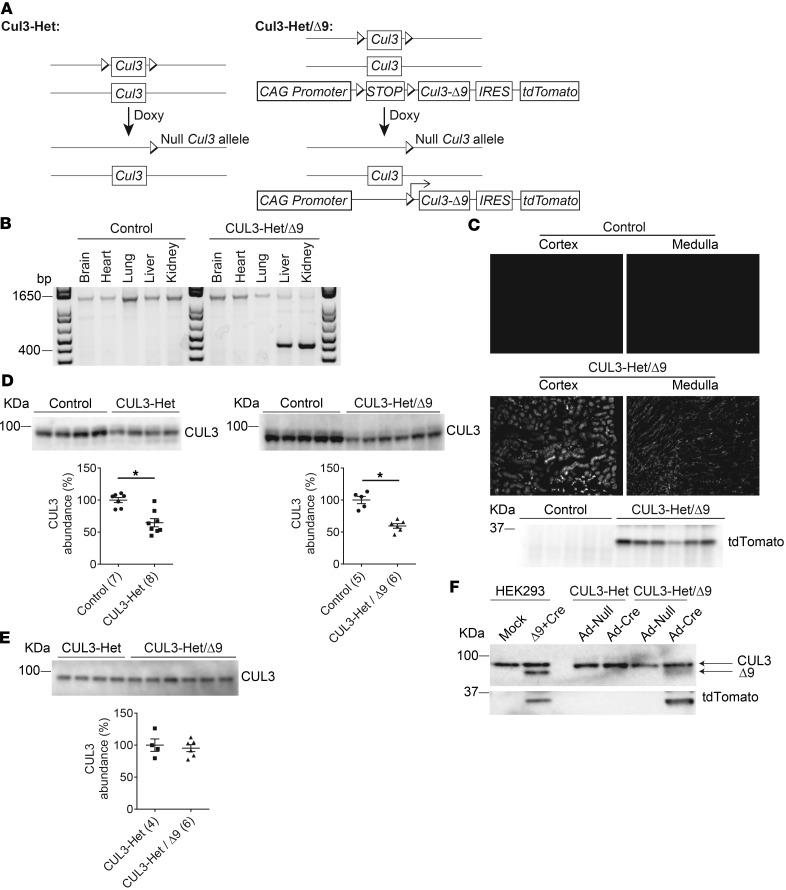
Figure 1. Generation of CUL3-Het and CUL3-Het/Δ9 mice.
(A) The Pax8-rtTA-LC1 system (present in all mice) was used for doxycycline-induced Cre-mediated recombination at loxP sites specifically in renal epithelia. Cul3 heterozygous mice (CUL3-Het mice, left) carried 1 WT Cul3 allele and a Cul3 allele with exons 4–7 floxed. CUL3-Het/Δ9 mice (right) additionally carried a transgene fragment consisting of a strong synthetic promoter (CAG) promoter, a floxed transcription blocker (STOP), cDNA encoding Cul3 Δ403-459 (CUL3-Δ9), an internal ribosome entry site (IRES), and cDNA encoding the fluorophore tdTomato; the IRES permitted translation of CUL3-Δ9 and tdTomato separately from the same transcript. Doxycycline (Doxy, 2 mg/ml in 5% sucrose drinking water vehicle for 2 weeks) treatment induced recombination, disrupting 1 Cul3 allele in both CUL3-Het and CUL3-Het/Δ9 mice, and — in CUL3-Het/Δ9 mice only — initiating expression of CUL3-Δ9 and tdTomato. Control mice were genetically identical to either CUL3-Het or CUL3-Het/Δ9 mice and were given vehicle only. (B) To confirm recombination in CUL3-Het/Δ9 mice following Doxy treatment, PCR was performed on genomic DNA from several tissues. In control mice, a 1,300 bp product, consistent with no recombination, was detected in brain, heart, lung, liver, and kidney. In CUL3-Het/Δ9 mice, a 430 bp product derived from the recombined transgene was detected in kidney and liver, but not in brain, heart, or lung. Pax8-rtTA activity in liver has been reported previously (33). (C) tdTomato fluorescence was observed in renal cortex and medulla in CUL3-Het/Δ9 mice, but not in controls (or CUL3-Het mice; data not shown), representative of 8 independent experiments, and Western blot of whole kidney confirmed transgene activation following recombination. (D) Western blot of whole kidney lysate showed that both CUL3-Het (*P = 0.02, 2-tailed unpaired t test) and CUL3-Het/Δ9 (*P = 0.0001, 2-tailed unpaired t test) mice had significantly reduced WT Cullin 3 (CUL3) abundance compared with controls. For quantification, densitometric values were normalized using Coomassie stained gels (see Supplemental Figure 1 for details); values in parentheses represent n. (E) Direct comparison of CUL3-Het and CUL3-Het/Δ9 mice confirmed no difference in CUL3 abundance. (F) Western blot of cell lysates from primary renal epithelial cells infected with empty adenovirus (Ad-Null) or Cre adenovirus (Ad-Cre) confirmed Cre-mediated CUL3-Δ9 expression. Primary cells from uninduced CUL3-Het and CUL3-Het/Δ9 mice were then infected with virus for 48 hours. The short infection time was not sufficient to reduce endogenous CUL3 abundance. Lysates from HEK293 cells transfected with plasmids encoding the transgene (Δ9) and Cre confirmed the size of CUL3-Δ9 in primary cells.