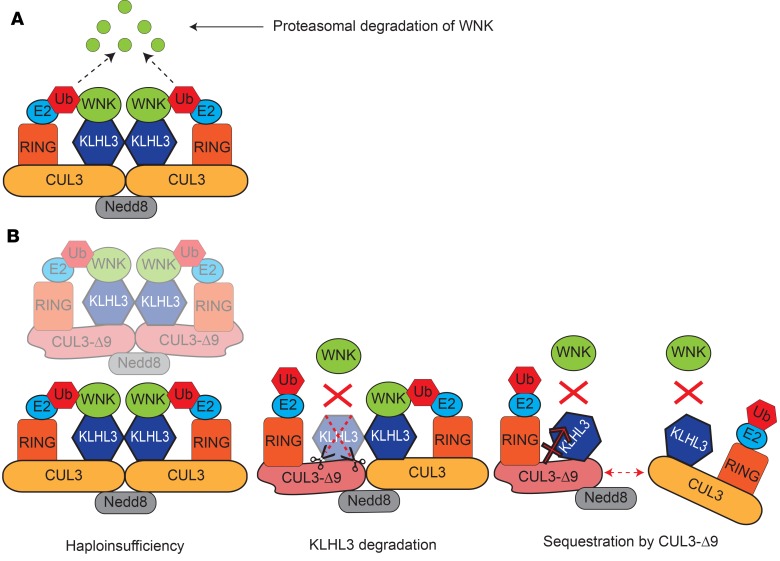
Figure 7. Model of normal Cullin RING Ligase (CRL) function and proposed mechanisms of CUL3-Δ9–mediated familial hyperkalemic hypertension (FHHt).
(A) The active WT CRL is a complex composed of 2 Cullin 3–containing (CUL3-containing) complexes. CUL3 acts as a scaffold for the complex. One CUL3 monomer is covalently linked to Neural precursor cell expressed developmentally downregulated protein 8 (Nedd8), which may facilitate dimerization with a non-Nedd8–conjugated CUL3 monomer and also activates the CRL (35). Each CUL3 monomer can interact with many different substrate adaptors, but Kelch-like 3 (KLHL3 is shown here since it is relevant to FHHt. WNK kinases interact with KLHL3, which brings them close to the RING E2 ubiquitin ligase that covalently attaches ubiquitin (Ub) to With-No-Lysine [K] Kinases (WNKs), targeting them for proteasomal degradation. (B) Three mechanisms have been proposed to explain how CUL3-Δ9 causes FHHt. In the haploinsufficiency model, CUL3-Δ9 triggers degradation of itself, but not of WT CUL3, by the proteasome (30). Fewer functional CRLs are available to degrade WNKs, which accumulate, leading to inappropriate Na+-Cl– cotransporter (NCC) phosphorylation and activation by Sterile 20 (STE20)/SPS-1–related proline/alanine-rich kinase (SPAK)/oxidative stress–response kinase-1 (OSR1). Our data do not support this model. In the KLHL3 degradation model, CUL3-Δ9 inappropriately degrades KLHL3, decreasing the number of active CRLs and causing accumulation of WNKs (28). Since KLHL3 is highly expressed along the distal convoluted tubule, the site of NCC expression, this leads to FHHt, which is primarily a disease of NCC dysregulation. This model assumes that CUL3-Δ9 exerts most of its effects on KLHL3 and not on other CUL3 adaptors. Western blotting (30) and immunofluorescence for KLHL3 (Supplemental Figure 10) in CUL3-Δ9–expressing mouse models do not support this model. In the sequestration model, CUL3/CUL3-Δ9 heterodimers are less stable than CUL3/CUL3 homodimers, and CUL3-Δ9 may also sequester adapters (29), leading to a lower number of active CRLs and resulting in WNK accumulation. Our data do not refute this model, but more extensive disruption of CRL activity might be expected to cause multiple defects, not just FHHt.