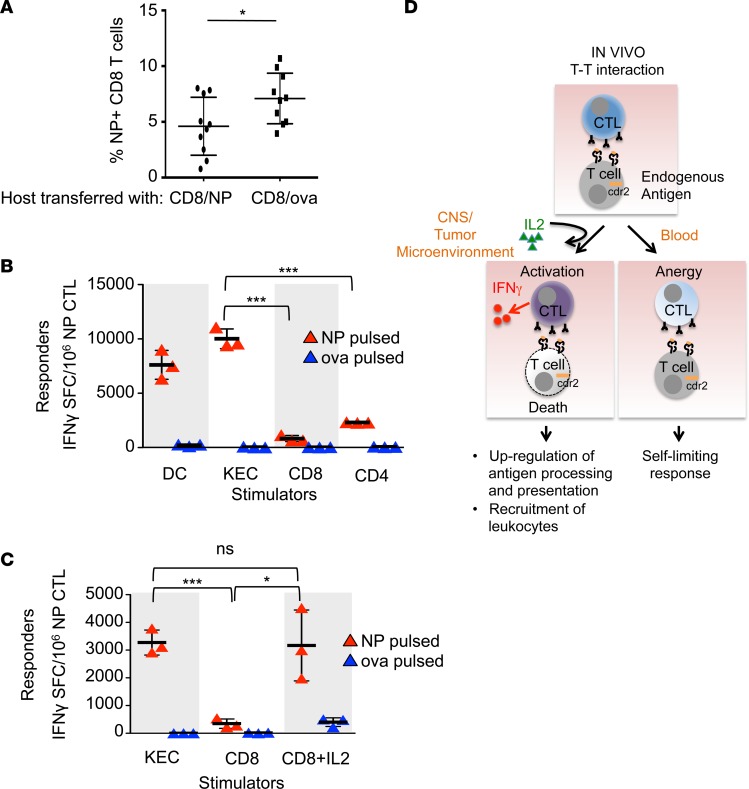
Figure 6. T cell antigen presentation induces effector CTL split anergy.
(A) Flow cytometry of circulating CD45.2+ NP-specific tetramer+ CD8+ T cells in a flu-infected host transferred with either NP-pulsed (n = 10) or OVA-pulsed (n = 10) CD45.1+ CD8+ T cells. Each bar represents the mean and error bars are standard deviations. These data are representative of 2 experiments. (B) IFN-γ ELISPOT assay of NP-specific effector CTLs cultured with DCs or kidney epithelial cells (KECs) (stimulator to effector ratio of 1:30), or CD8+ or CD4+ T cells (stimulator to effector ratio of 1:1). Each bar represents the mean of triplicate wells and error bars are standard deviations. These data are representative of 2 experiments. (C) IFN-γ ELISPOT assay of NP-specific effector CTLs cultured with KECs (stimulator to effector ratio of 1:30), CD8+ T cells (stimulator to effector ratio of 1:1), or CD8+ T cells plus IL-2. Stimulator cells were pulsed with either NP or OVA peptide. Each bar represents the mean of triplicate wells and error bars are standard deviations. These data are representative of 2 experiments. *P < 0.05, ***P < 0.001; ns, statistically not significant as calculated using unpaired Student’s t test. (D) Proposed model of the plasticity of CTL responses to antigen encounters and how the data presented in this paper may relate to human diseases. In the steady state (peripheral blood), armed effector CTLs respond to other T cells presenting cognate antigen by killing the stimulator T cell, but do not secrete IFN-γ. The effector CTL itself survives and is anergic. In the context of an inflammatory microenvironment (tumor or other inflamed tissue), exogenous IL-2 licenses the effector CTL to become fully activated and it secretes IFN-γ in addition to killing targets. IFN-γ augments immune responses upregulating antigen processing and presentation as well as promoting the recruitment of leukocytes to the tissue, promoting tumor immunity and autoimmunity seen in paraneoplastic neurologic disease. SFC, spot-forming cells.