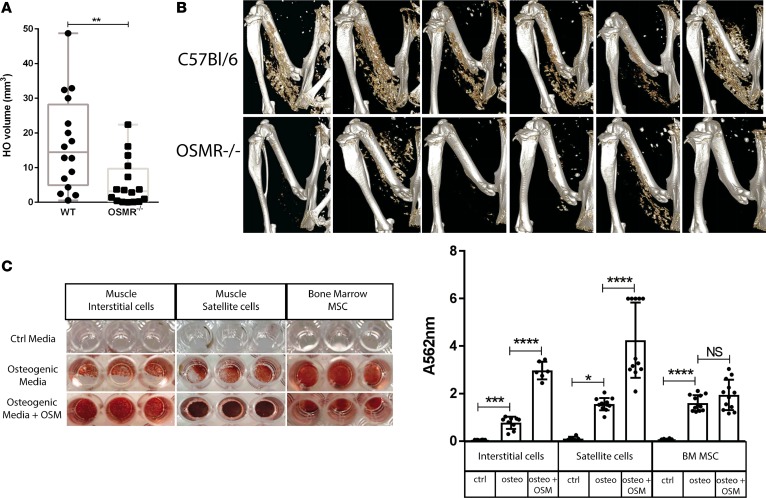
Figure 9. Deletion of the Osmr gene reduces NHO following SCI in mice.
(A) μCT analysis of neurogenic heterotopic ossification (NHO) development 7–14 days following spinal cord injury (SCI) and intramuscular cardiotoxin (CDTX) injection in oncostatin M receptor null (Osmr–/–) mice compared with wild-type C57BL/6 control mice. Each dot represents an individual mouse, and the box-and-whisker plot shows median, 25th and 75 percentile, and minimum and maximum values. Statistical significance was confirmed using a Mann-Whitney test (P = 0.0038, 16 mice/group, experiment repeated twice). (B) Representative 3D reconstructed images of NHO in C57BL/6 and Osmr–/– mice 7 days after surgery. (C) Recombinant mouse oncostatin M (OSM) enhances in vitro osteogenic differentiation. Muscle interstitial cells, satellite cells, and bone marrow mesenchymal stromal cells (BM-MSCs) sorted from naive C57BL/6 mice were cultured for 10 days in control medium, osteogenic medium, or osteogenic medium plus mouse OSM (25 ng/ml). Quantification of Alizarin Red S staining via absorbance at 562 nm confirmed enhanced calcium deposition in interstitial cells (***P < 0.01), satellite cells (*P < 0.05), and BM-MSCs (****P < 0.001) in the presence of osteogenic media alone and significantly enhanced calcium deposition in both interstitial cells and satellite cells in the presence of osteogenic media plus OSM (****P < 0.001). Results are the mean and SDs of 2 separate experiments (n = 3 control media and n = 3–6 osteogenic media ± OSM/each experiment).