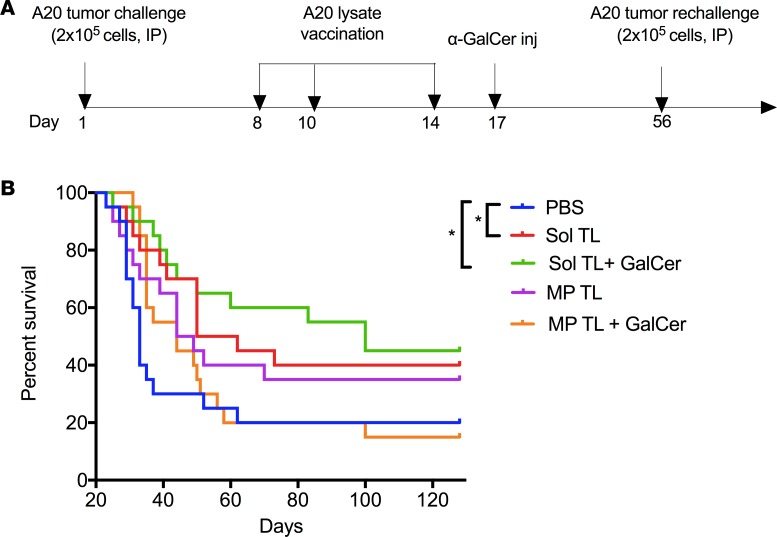
Figure 2. Therapeutic efficacy of soluble and microparticle-encapsulated A20 lysate vaccine formulations in 7-day preestablished A20 B-lymphoma model.
(A) Time line for therapeutic immunization and tumor rechallenge studies in 7-day preestablished A20 B cell lymphoma model. (B) Kaplan-Meier survival curve (n = 20 per group, pooled data from 2 independent experiments) for various A20 tumor lysate formulation–treated mice after 2 lethal A20 tumor challenges of 2 × 105 cells/mouse. Naive BALB/C mice were first injected (i.p.) with a lethal dose of A20 cells (2 × 105 cells/mouse), followed by 3 immunizations at days 8, 10, and 14 with various tumor lysate formulations (100 μg lysate protein/mouse, s.c.) and at day 17 with α-GalCer (2 μg/mouse, i.p.). A second challenge with 2 × 105 A20 tumor cells/mouse, i.p., was done at day 56, and moue survival was followed up to 128 days. *P < 0.05, 1-way ANOVA with Tukey multiple comparison tests.