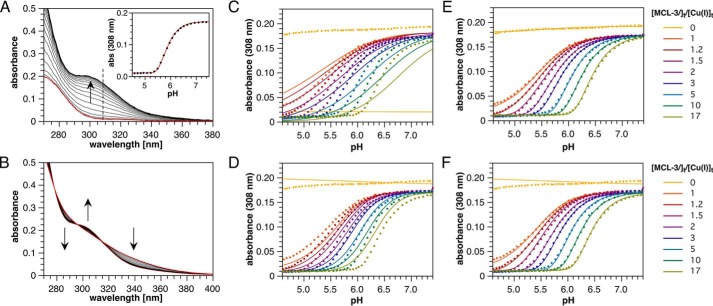
Figure 4.
Spectrophotometric pH titration data and global non-linear least-squares fitting results for the Cu(I)-glutathione equilibrium system using MCL-3 as competitor ligand (100 μm Cu(I), 4 mm GSH, and universal buffer, 0. 1 m KCl, 25 °C). A, representative UV absorption spectra for a pH titration in the presence of 290 μm MCL-3. The red trace was acquired at pH 4.5 where Cu(I) is complexed to MCL-3 only. Inset, non-linear least squares fit shown for the pH-dependent absorption change at 308 nm. B, UV-visible absorption spectra for a pH titration in the absence of MCL-3. The arrows indicate the direction of spectral changes with increasing pH. C–F, data (solid circles) and global non-linear least square fit (continuous lines) for 18 independent pH titrations in the presence of 0 to 17 molar eq of MCL-3 relative to total Cu(I). For clarity, absorption data and fitted traces are shown only at 308 nm as a function of pH (for comprehensive residual plots see Fig. S3). Equilibrium models and fitted parameters are as follows: [Cu(GS)] with logβ11 = 15.42 (χ2 = 7.885) (C); [Cu(GS)2] with logβ12 = 20.97 (χ2 = 2.705) (D); [Cu4(GS)6], [Cu5(GS)7], and [Cu5(GS)6], with logβ46 = 84.92, logβ56 = 97.99, logβ57 = 103.92, (χ2 = 0.424) (E); and [Cu4(GS)6] with logβ46 = 84.97 (χ2 = 1.029) (F).