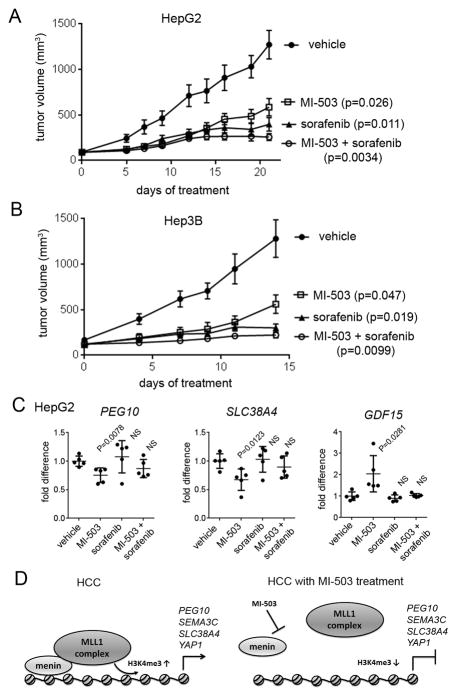
Figure 6. In vivo efficacy of MI-503 in HCC models.
A, B. In vivo inhibition of the tumor growth by MI-503 (35 mg/kg, i.p.), sorafenib (20 mg/kg or 40 mg/kg in HepG2 and Hep3B xenografts, respectively, p.o.) or combination of both agents relative to vehicle control in HepG2 (A) and Hep3B (B) mice xenograft models, n = 8–9 mice per group. Mice were treated once daily at the doses indicated above. Error bars represent SEM. C. Expression of PEG10, SLC38A4 and GDF15 measured by qRT-PCR of RNA extracted from tumor samples harvested at the end point of treatment with MI-503, sorafenib, combination of both agents or vehicle in HepG2 xenograft model (panel A). PEG10, SLC38A4 and GDF15 transcripts levels are normalized to the mean transcript level in tumors from vehicle treated mice; n = 5 per group. p values < 0.05 are considered significant. NS – not significant. D. Menin interaction with the MLL1 histone methyltransferase complex leads to increase in the H3K4 tri-methyl mark on PEG10 and possibly other genes relevant to HCC resulting in increased expression of these genes (left panel). Inhibition of the menin-MLL1 interaction by MI-503 results in reduced H3K4me3 and repression of PEG10 and possibly other genes relevant to HCC (right panel).