Figure 3. Overall potential actionability of characterized alterations based on available agents.
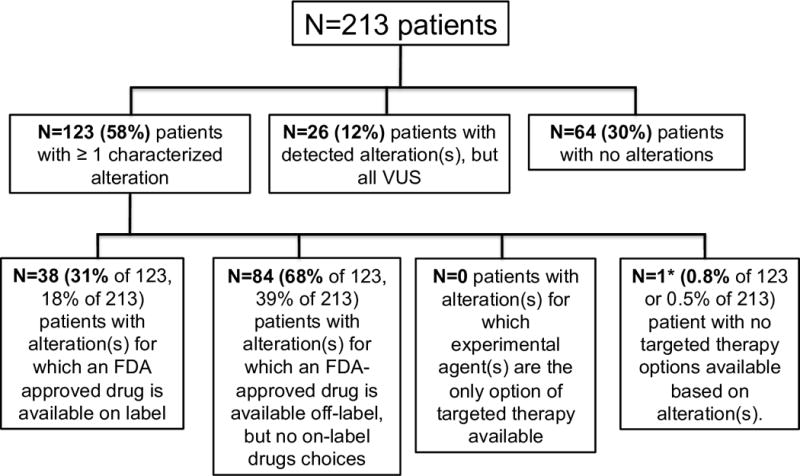
Almost all patients (122/123) with characterized alterations(s) had one or more targeted therapy(ies) available, either through an on-label drug, an off-label drug, or a clinical trial. For patients who had multiple therapies available, FDA approved agents were prioritized over experimental drugs. Amongst FDA approved agents, on-label drugs were prioritized over off-label drugs.
