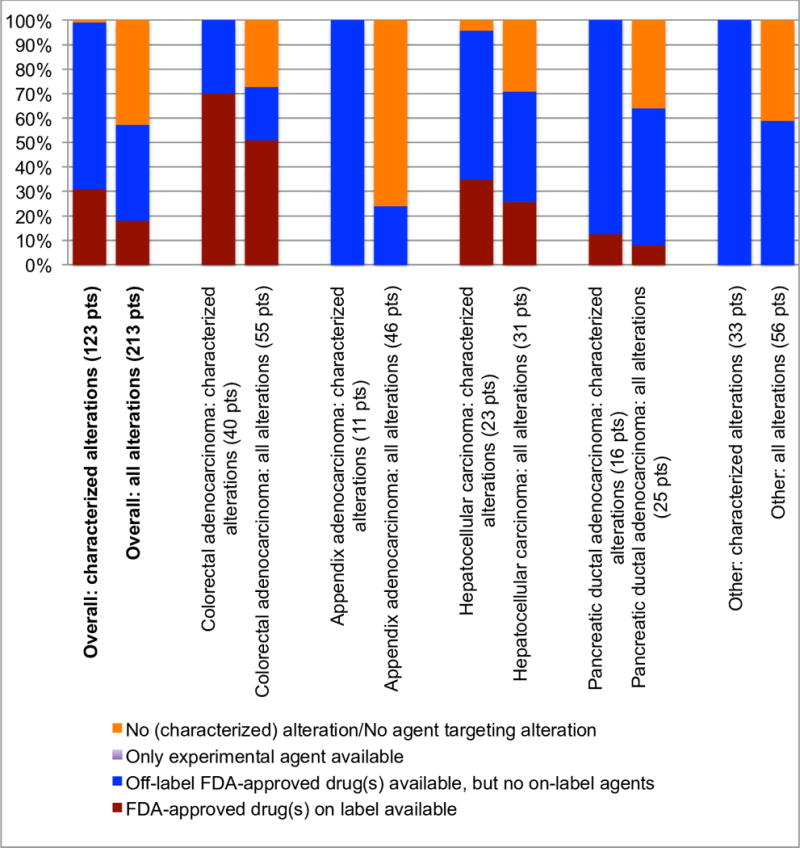
Figure 4. Theoretical treatment options for characterized alterations across diverse gastrointestinal cancers.
Abbreviations: FDA = Food and Drug Administration; pts = patients
Excluding variants of unknown significance, the percent breakdown of available treatment options for patients with the most common cancer-types in the cohort. FDA approved agents were prioritized over experimental drugs. Amongst FDA approved agents, on-label drugs were prioritized over off-label drugs. In our population, all of the patients who were candidates for an experimental agent also had targeted FDA-approved drugs available, either on or off-label (see Figure 3).
