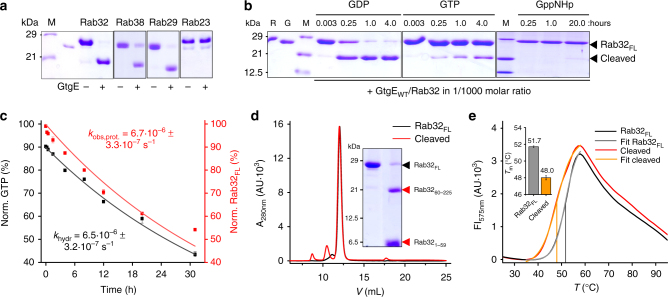
Fig. 1.
GtgE specifically cleaves the inactive form of Rab32-family members. a Substrate range of GtgE is restricted to Rab32 family members. Proteolytic gel shift assay of the Rab32-subfamily and Rab23. b Nucleotide-dependent GtgE-mediated (G, 8 nM) cleavage of Rab32FL (R, 8 µM) bound to GDP, GTP or GppNHp in a time-dependent SDS-PAGE gel shift assay. c Direct comparison of the intrinsic GTP-hydrolysis of Rab32:GTP (black) and the GtgE-mediated proteolysis of Rab32:GTP (red). GTP-hydrolysis was monitored by quantifying the GTP-content using ion-pairing reversed-phase chromatography (Supplementary Fig. 2A). The proteolysis was monitored by Coomassie-stained SDS-PAGE (Supplementary Fig. 2B). Both data sets are presented as means of technical replicates ( ± SEM; n = 2). The data were fitted to a single exponential function, yielding the observed rate constants k obs,prot and k hydr. d Proteolytic cleavage does not promote unfolding of Rab32. Comparison of the retention times of Rab32FL (8 µM, black) and cleaved Rab32 (8 µM, red) by size-exclusion chromatography (SEC). Inset: Full conversion was verified by SDS-PAGE. e Rab32 cleavage mildly affects thermal stability. Melting point (T m) determination of Rab32FL (black) and cleaved Rab32 (red) by differential scanning fluorimetry (DSF). The data were fitted to a Boltzmann equation, yielding the melting temperature. Inset: Results are presented as bar graphs with means ± SEM (n = 3)