Fig. 5.
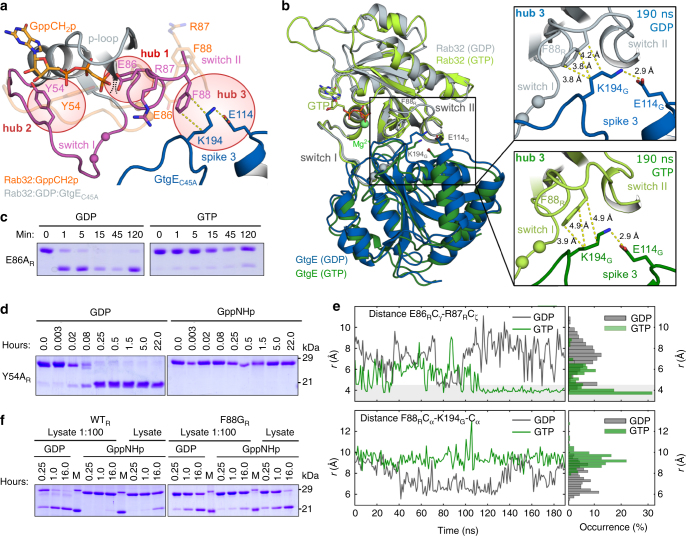
A molecular basis of the GDP-state specificity of GtgE. a Putative hubs involved in the GDP-state specificity of GtgE depicted in the superposition of active and inactive Rab32-structures. Gray: Rab32:GDP:GtgEC45A; transparent wheat: Rab32:GppCH2p:VARP (PDB ID: 4CYM17); sticks: Hub 1 (E86R), hub 2 (Y54R), hub 3 (comprising of F88R, E114G, and K194G), and GppCH2p; spheres: Cα-atoms of the cleavage site; magenta: switch regions Rab32:GDP; black and yellow dashed lines: steric clash and hydrogen bonds, respectively. b Hub 3 (F88R; E114G; K194G) of the Rab:GtgEC45A switch II interface. Structural superposition of MD-simulations of the Rab32:GtgE complex bound to either GDP (gray/blue) or GTP positioned in silico (light/dark green) after 190 ns. Right: van-der-Waals contacts of hub 3 indicated by yellow dashed lines for the GDP (top) and the GTP-complex (bottom), respectively (see Supplementary Fig. 18). c, d Processing of the hub mutants hub 1 E86AR (c), and hub 2 Y54AR (d) by GtgE in the GDP and GTP states, analyzed with a gel shift activity assay (see also Supplementary Fig. 15A). e Selected atom pair distances in a GDP-bound and hypothetical GTP-bound Rab32:GtgE complex from 190 ns MD-simulations (left panels) and histograms of respective distance occurrences (right panels), with an ion-pair distance threshold (< 4.5 Å) highlighted in gray. Top: E86R and R87R form a stable ion-pair after ca. 120 ns in the hypothetical GTP-bound complex (in green), initiated by an electrostatic repulsion from the γ-phosphate of GTP (see also Supplementary Fig. 18C). In contrast, no stable ion-pair between E86R and R87R is observed in the GDP-bound structure (in gray). Bottom: Distances between F88R of Rab32 and K194G of GtgE from MD-simulations of the GDP- (in gray) and GTP-bound (in green) Rab32:GtgE complexes. In the GDP-bound form, K194G forms an interaction with F88R, while in the GTP-bound complex, K194G dissociates from F88R, which may trigger the dissociation of GtgE from Rab32 (see also Supplementary Fig. 18A). The MD-simulations suggest that F88R may form a decisive element for the GDP-state preference of GtgE toward Rab32. f Mutations in Rab32 hub 3 change GtgEs nucleotide-state selectivity leading to cleavage of Rab32:GppNHp. Rab32 F88G loaded with GDP or GppNHp was treated with cleared E. coli lysate (or 1:100 diluted lysate) overexpressing wild-type GtgE and analyzed by an gel shift assay (see also Supplementary Fig. 18B)