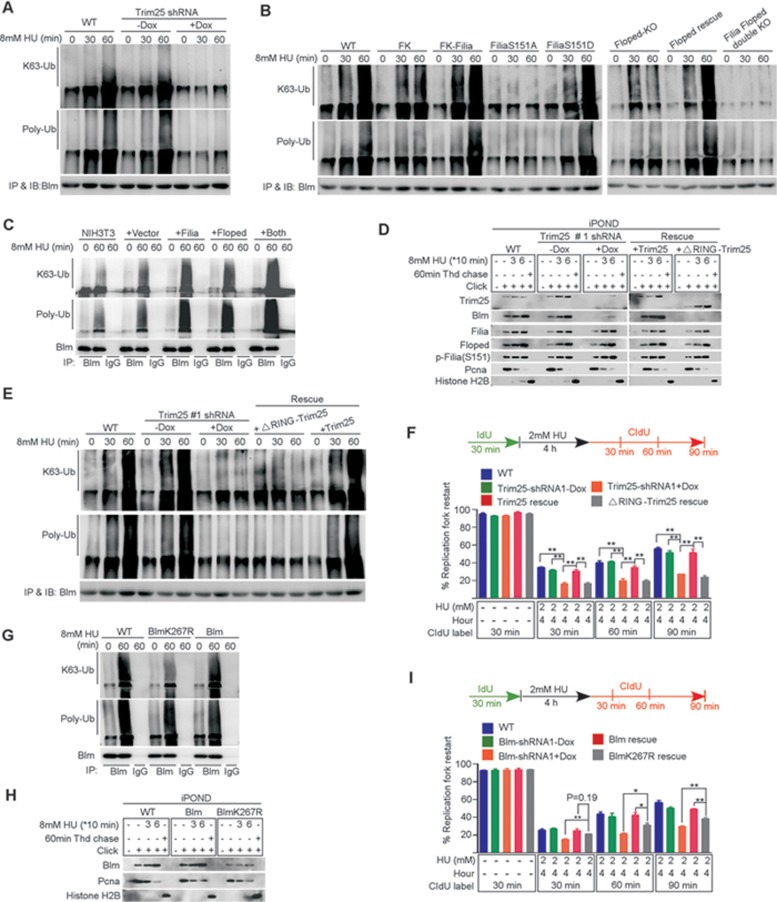
Figure 7.
Trim25 ubiquitinates Blm to promote the recruitment of Blm to replication forks. (A) Blm ubiquitination was significantly increased by the HU treatment in WT ESCs. However, knockdown of Trim25 significantly suppressed this modification. (B) In Filia-knockout (FK), Floped-knockout or double knockout ESCs, the ubiquitination level of Blm was lower than that in WT ESCs. Re-expression of WT Filia, Floped or FiliaS151D restored the Blm ubiquitination. However, expression of FiliaS151A into FK cells failed to rescue the defect. (C) Ectopic expression of Filia, Floped or Filia plus Floped in NIH3T3 cells increased the Blm ubiquitination level. (D) ΔRING-Trim25 mutant protein was normally localized on replication forks. However, it could not recruit Blm to replication forks. (E) ΔRING-Trim25 failed to regulate the ubiquitination of Blm. (F) ΔRING-Trim25-expressing ESCs were as defective as Trim25-knockdown ESCs in restarting stalled replication forks. (G) BlmK267R mutation impaired the K63-linked poly-ubiquitination of Blm in response to HU treatment in mESCs. (H) BlmK267R mutation compromised Blm recruitment to replication forks. (I) The ability to restart replication forks in BlmK267R-complemented ESCs was lower than in WT Blm-rescued cells, but higher than in Blm-knockdown ESCs. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01.