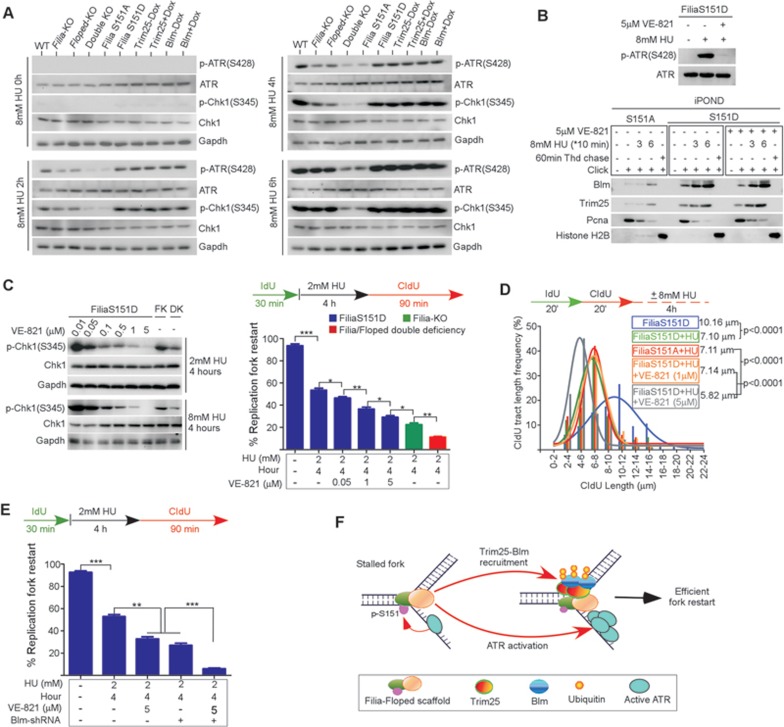
Figure 8.
Filia-Floped scaffolds promote ATR activation independent of the Trim25-Blm pathway to regulate stalled fork restart. (A) Time-course analysis of ATR activation in response to HU treatment. Deficiency of Filia or Floped impaired ATR activation, double deficiency or FiliaS151A mutation caused further inhibition. However, depletion of Trim25 or Blm had no influence on ATR activation. (B) Inhibition of ATR activity in FiliaS151D ESCs did not affect the recruitment of Trim25 and Blm to stalled replication forks. (C) ATR activity was suppressed to different extents in FiliaS151D ESCs to determine its influence on stalled fork restart and nascent DNA degradation. ATR signaling regulated stalled fork restart in a dose-dependent manner. Filia-knockout (FK) ESCs and Filia-Floped double deficient (DK) cells were used as controls. (D) However, nearly complete inhibition of ATR signaling was required for nascent DNA degradation. (E) Individual inhibition of ATR activation or Blm protein expression in FiliaS151D-complemented ESCs decreased stalled fork restart ability. Simultaneous inhibition of both pathways caused further suppression of fork restart. (F) Working model of Filia-Floped scaffold in promoting stalled fork restart in ESCs.