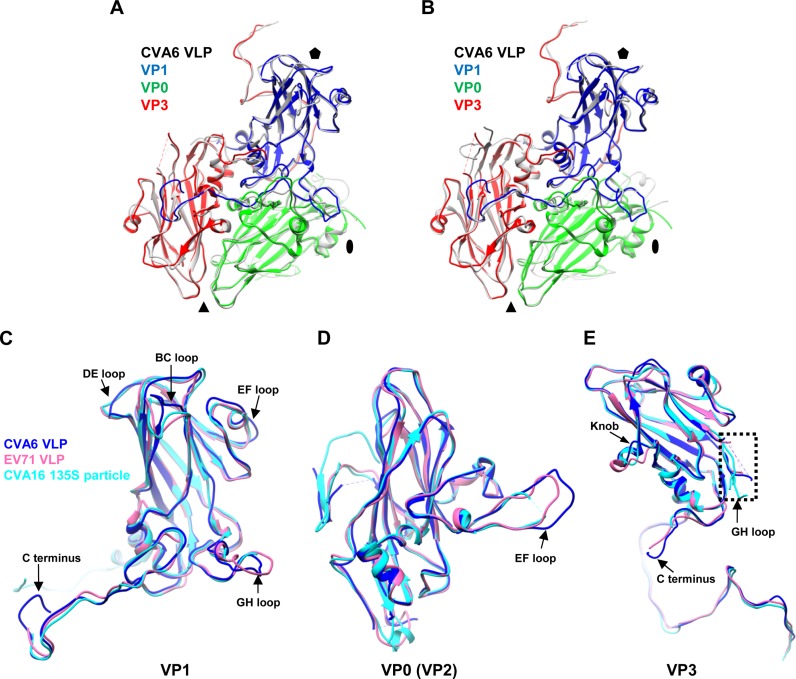
FIG 6.
Structural comparison of the protomers and individual capsid proteins of the CVA6 VLP, EV71 VLP, and CVA16 135S particle. (A) Superposition of the protomers of the CVA6 VLP (different capsid proteins in different colors) and EV71 VLP (gray). The black oval, triangle, and pentagon represent the 2-fold, 3-fold, and 5-fold axes, respectively. (B) Superposition of the protomers of the CVA6 VLP and CVA16 135S particle (gray). VP1, VP0, and VP3 of CVA6 VLP are in blue, green, and red, respectively. (C to E) Superposition of individual capsid protein structures of the CVA6 VLP (blue), EV71 VLP (pink), and CVA16 135S particle (cyan). (C) Superposition of VP1. (D) Superposition of VP0/VP2 (VP0 for the CVA6 VLP and EV71 VLP and VP2 for the CVA16 135S particle). (E) Superposition of VP3. The major structural differences of subunit proteins among the CVA6 VLP, EV71 VLP, and CVA16 135S particle are indicated by black arrows. The dashed rectangle indicates the disordered region of the VP3 GH loop.