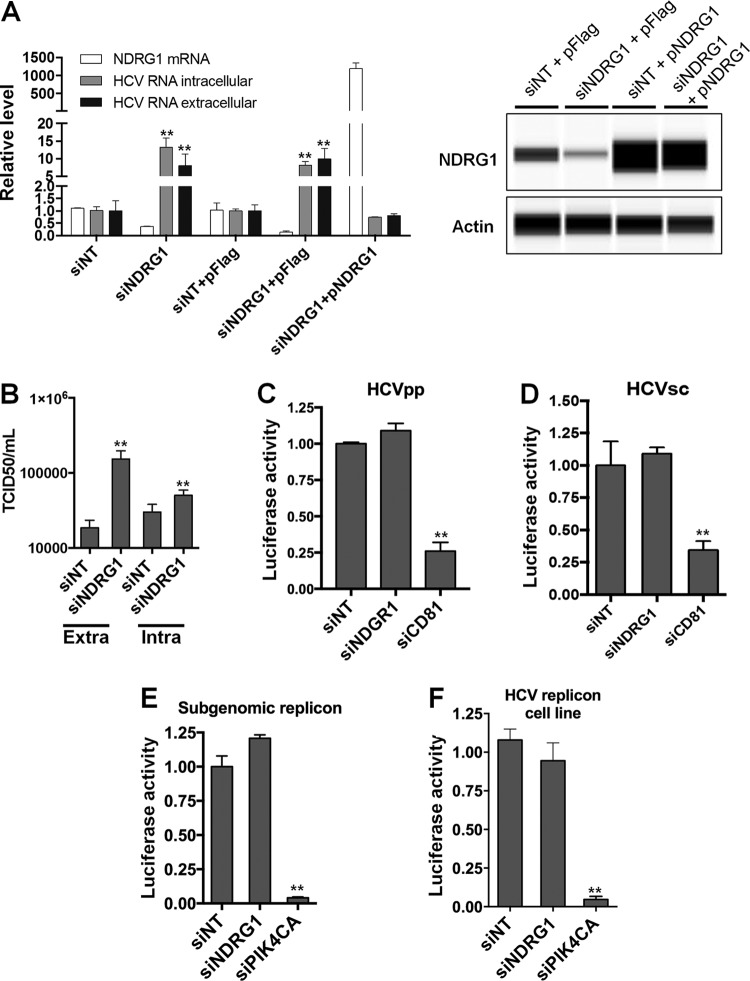
FIG 1.
Loss of NDRG1 enhances HCV infection. (A) Knockdown of NDRG1. Huh7.5.1 cells were transfected with a pool of NDRG1-specific, no-target control siRNAs (siNT), and/or the NDRG1 plasmid (pNDRG1) for 72 h. Cells were infected with HCV for 48 h, and total intracellular and extracellular RNA was isolated and quantified by RT-qPCR using gene-specific primers. NDRG1 knockdown and overexpression were also detected by Western blotting using the Wes system. (B) HCV assembly is increased in NDRG1-depleted cells. Huh7.5.1 cells were treated as described above for panel A and infected with HCV. At 48 h postinfection, extracellular and intracellular virus was isolated and used to infect naive Huh7.5.1 cells. Infectivity (50% tissue culture infective dose [TCID50] per milliliter) was calculated by limiting dilution and detection of the HCV core protein by immunofluorescence. (C and D) NDRG1 depletion does not affect HCV entry. Knockdown in Huh7.5.1 cells was carried out as described above, and two different viral luciferase reporter constructs were utilized to examine entry (HCVpp and HCVsc). Cells were lysed, and luciferase activity was measured 48 h later by using a luminometer. CD81 knockdown was used as a positive control for entry. (E and F) NDRG1 knockdown does not alter HCV RNA replication. Huh7.5.1 cells were treated with siRNA for 72 h and transfected with the HCV SGR (E), while Huh7-SGR cells (F) were treated with siRNAs only. Knockdown of PI4KCA was used as a positive control in each system. Luciferase activity was measured 72 h after treatment. All error bars denote standard deviations. All experiments were performed three times, and the data from a representative experiment are shown. **, P ≤ 0.01; *, P ≤ 0.05 (comparison to the negative controls).